Kinetic Molecular Theory Number of molecules Temp Volume
Kinetic Molecular Theory • • Number of molecules Temp Volume Pressure • • Number of dancers Beat of music Size of room Number and force of collisions Mullis 1
Kinetic Molecular Theory • Accounts for behavior of atoms and molecules • Based on idea that particles are always moving • Provides model for an ideal gas • Ideal Gas = Imaginary: Fits all assumptions of the K. M theory • Real gas = Does not fit all these assumptions Mullis 2
5 assumptions of Kinetic-molecular Theory 1. Gases = large numbers of tiny particles that are far apart. 2. Collisions between gas particles and container walls are elastic collisions (no net loss in kinetic energy). 3. Gas particles are always moving rapidly and randomly. 4. There are no forces of repulsion or attraction between gas particles. 5. The average kinetic energy of gas particles depends on temperature. Mullis 3

Physical Properties of Gasses • Gases have no definite shape or volume – they take shape of container. • Gas particles glide rapidly past each other (fluid). • Gases have low density. • Gases are easily compressed. • Gas molecules spread out and mix easily Mullis 4
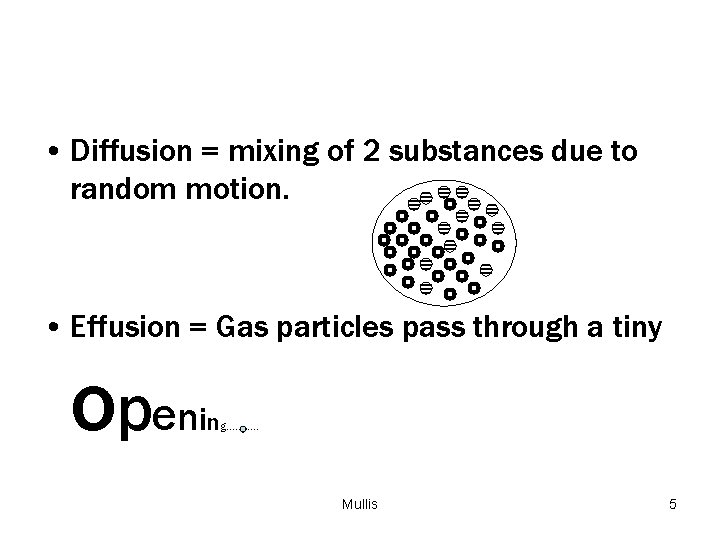
• Diffusion = mixing of 2 substances due to random motion. • Effusion = Gas particles pass through a tiny o p en in g……. . … Mullis 5

Real Gases • Real gases occupy space and exert attractive forces on each other. • The K-M theory is more likely to hold true for particles which have little attraction for each other. • Particles of N 2 and H 2 are nonpolar diatomic molecules and closely approximate ideal gas behavior. • More polar molecules = less likely to behave like an ideal gas. Examples of polar gas molecules are HCl, ammonia and water. Mullis 6

Gas Behavior • Particles in a gas are very far apart. • Each gas particle is largely unaffected by its neighbors. • Gases behave similarly at different pressures and temperatures according to gas laws. Mullis 7
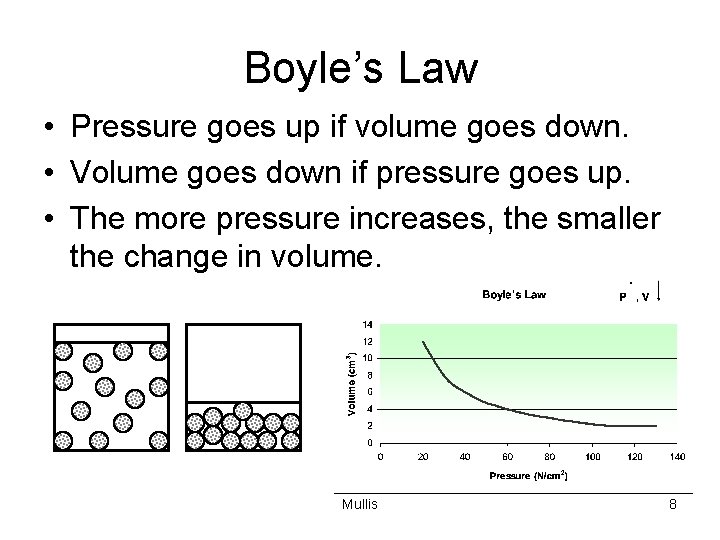
Boyle’s Law • Pressure goes up if volume goes down. • Volume goes down if pressure goes up. • The more pressure increases, the smaller the change in volume. Mullis 8
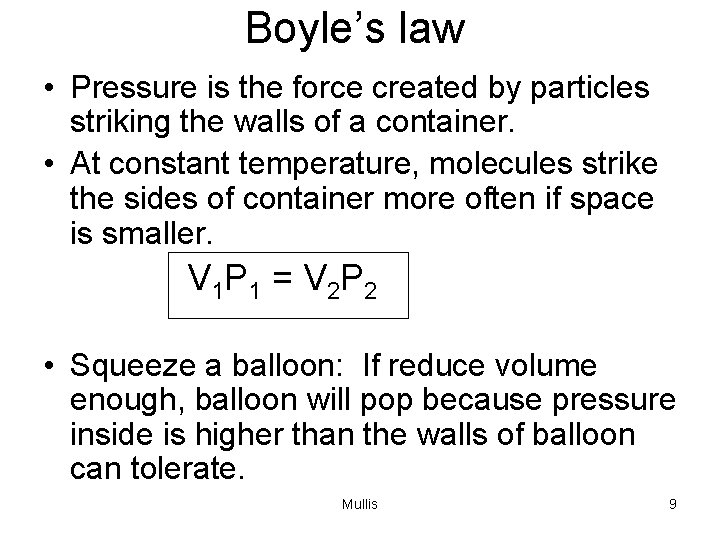
Boyle’s law • Pressure is the force created by particles striking the walls of a container. • At constant temperature, molecules strike the sides of container more often if space is smaller. V 1 P 1 = V 2 P 2 • Squeeze a balloon: If reduce volume enough, balloon will pop because pressure inside is higher than the walls of balloon can tolerate. Mullis 9
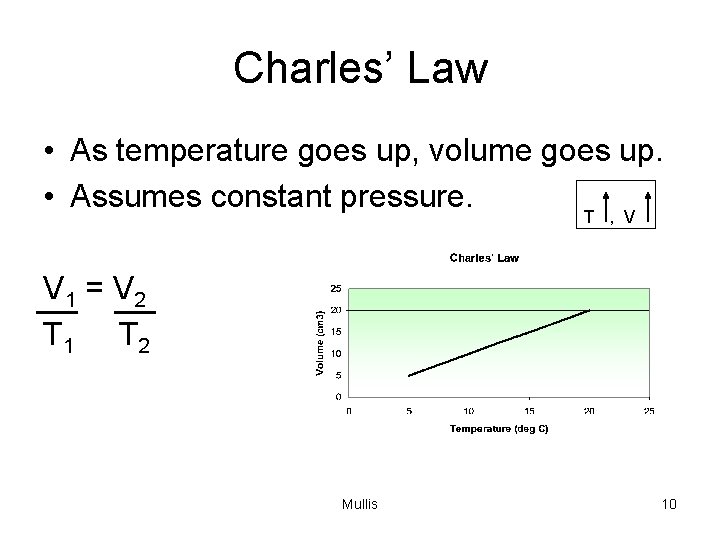
Charles’ Law • As temperature goes up, volume goes up. • Assumes constant pressure. T , V V 1 = V 2 T 1 T 2 Mullis 10

Charles’ law • As temperature goes up volume goes up. • Adding heat energy causes particles to move faster. • Faster-moving molecules strike walls of container more often. The container expands if walls are flexible. • If you cool gas in a container, it will shrink. • Air-filled, sealed bag placed in freezer will shrink. Mullis 11

Gay-Lussac’s Law • As temperature increases, pressure increases. • Assumes volume is held constant. P 1 = P 2 T 1 T 2 • A can of spray paint will explode near a heat source. • Example is a pressure cooker. Mullis 12
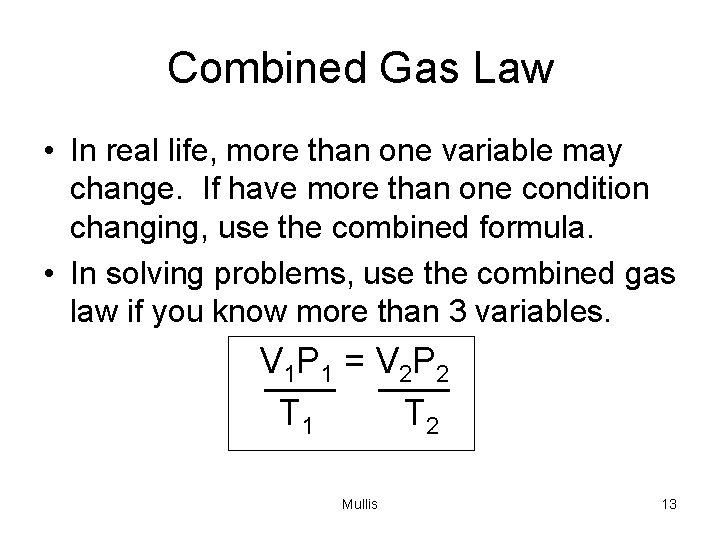
Combined Gas Law • In real life, more than one variable may change. If have more than one condition changing, use the combined formula. • In solving problems, use the combined gas law if you know more than 3 variables. V 1 P 1 = V 2 P 2 T 1 T 2 Mullis 13
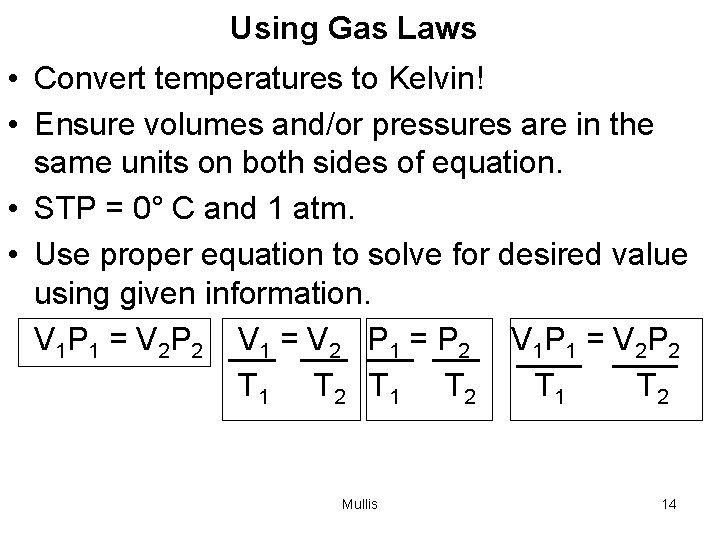
Using Gas Laws • Convert temperatures to Kelvin! • Ensure volumes and/or pressures are in the same units on both sides of equation. • STP = 0° C and 1 atm. • Use proper equation to solve for desired value using given information. V 1 P 1 = V 2 P 2 V 1 = V 2 P 1 = P 2 V 1 P 1 = V 2 P 2 T 1 T 2 Mullis 14
- Slides: 14