Kinetic model of a gas The kinetic model









- Slides: 16

Kinetic model of a gas • The kinetic model of a gas states that: • that the spacing of the particles is large compared to their size. • that the average kinetic energy of the particles depends on the temperature of the gas

What does this mean? Car tyres must be inflated to the correct pressure. What causes this pressure?

Pressure In terms of kinetic theory, pressure is caused by the force of particles hitting the walls of a container.

Pressure and Volume • What do you think would happen to the pressure of a gas if the volume of its container was reduced?

Conclusion As the of a gas volume increases its pressure decreases. Why does this happen?

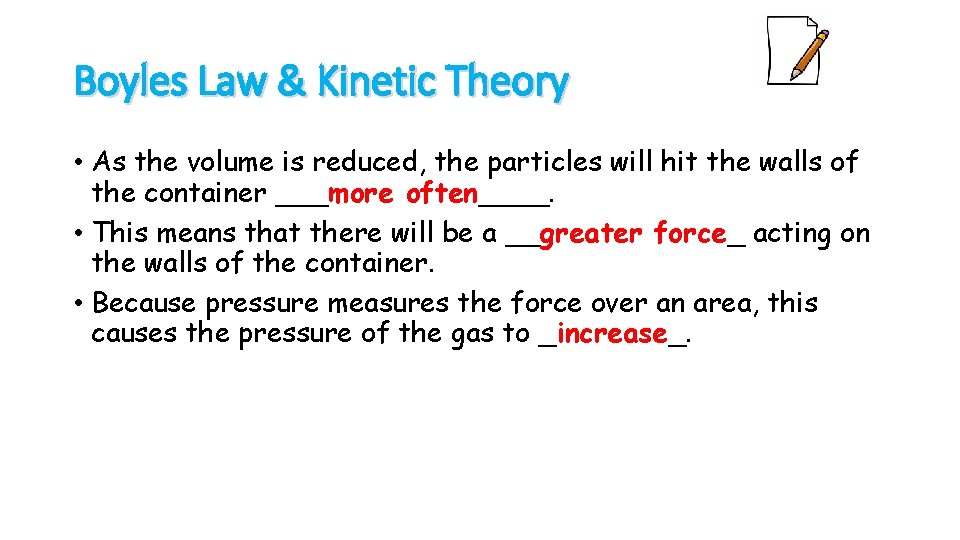
Boyles Law & Kinetic Theory • As the volume is reduced, the particles will hit the walls of the container ___more often____. • This means that there will be a __greater force_ acting on the walls of the container. • Because pressure measures the force over an area, this causes the pressure of the gas to _increase_.

Pressure & Volume 1. Using kinetic theory, explain step by step why a packet of crisps will burst when you squeeze it.

Pressure & Volume 2. Using kinetic theory, explain what happens to the marshmallow as the air is removed from the gas jar. https: //www. youtube. com/watch? v=DXQo 2 SAHp. F 8

Pressure and Temperature of a Gas What happens to the pressure of a gas as we increase the temperature? (think about what happens to sealed food containers when they are left out of the fridge) -> As temperature increases, pressure increases as a gas expands.

What does this mean? What happens to the pressure as the temperature increases?

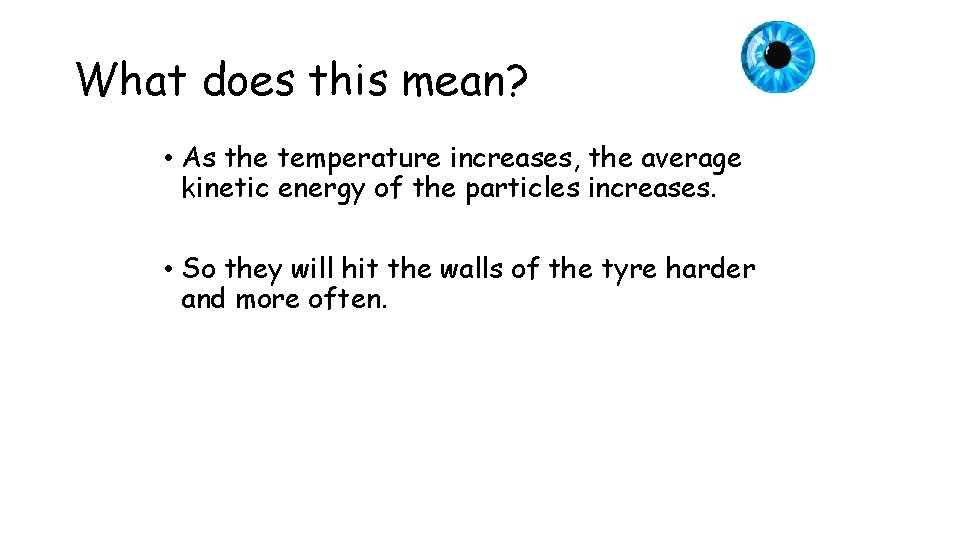
What does this mean? • As the temperature increases, the average kinetic energy of the particles increases. • So they will hit the walls of the tyre harder and more often.

Temperature and Pressure • After a long car journey on the motorway, the friction between the tyres and the road will have caused them to heat up. What will happen to the pressure?

Temperature and Pressure The pressure will increase!

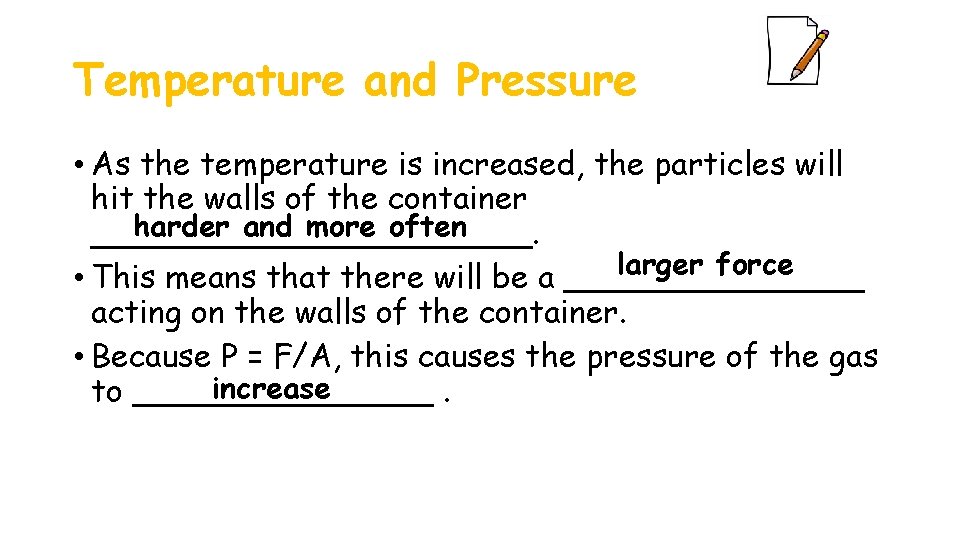
Temperature and Pressure • As the temperature is increased, the particles will hit the walls of the container harder and more often ___________. larger force • This means that there will be a ________ acting on the walls of the container. • Because P = F/A, this causes the pressure of the gas increase to ________.

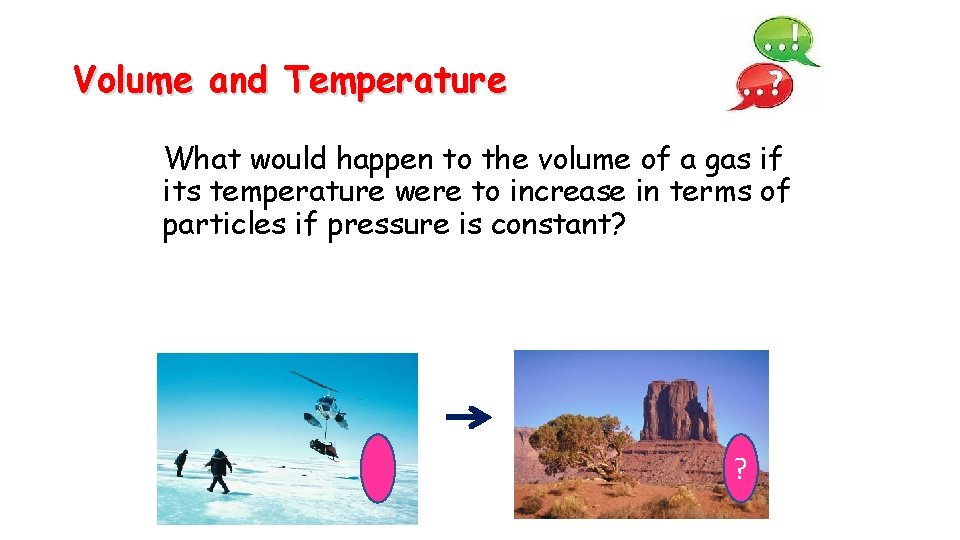
Volume and Temperature What would happen to the volume of a gas if its temperature were to increase in terms of particles if pressure is constant? ?

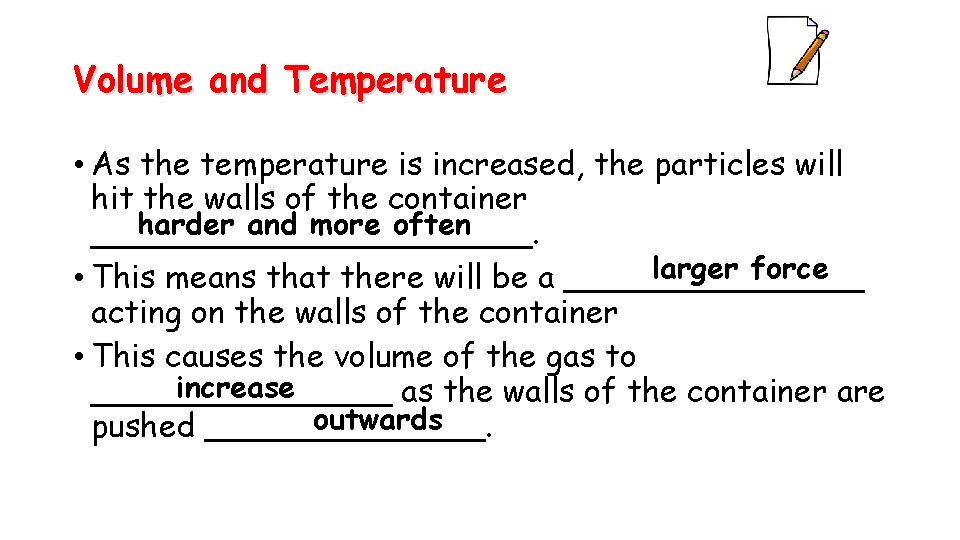
Volume and Temperature • As the temperature is increased, the particles will hit the walls of the container harder and more often ___________. larger force • This means that there will be a ________ acting on the walls of the container • This causes the volume of the gas to increase ________ as the walls of the container are outwards pushed _______.