Kinetic Energy and Changes of State Kinetic energy















- Slides: 41

Kinetic Energy and Changes of State

• Kinetic energy is the energy an object has b/c it is in motion. • Kinetic Theory states that all matter consists of tiny particles that are ALWAYS in motion.

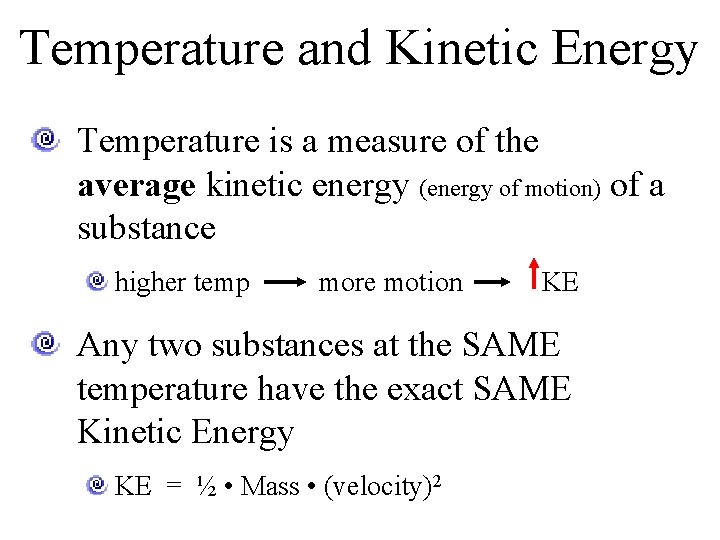
Temperature and Kinetic Energy Temperature is a measure of the average kinetic energy (energy of motion) of a substance higher temp more motion KE Any two substances at the SAME temperature have the exact SAME Kinetic Energy KE = ½ • Mass • (velocity)2

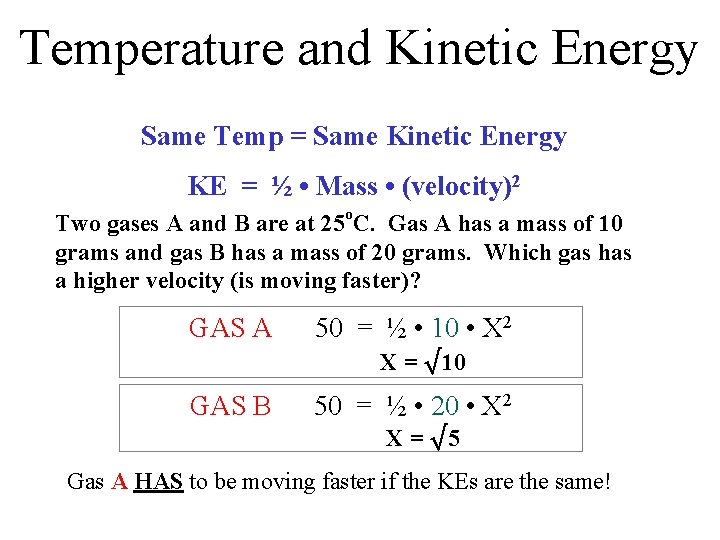
Temperature and Kinetic Energy Same Temp = Same Kinetic Energy KE = ½ • Mass • (velocity)2 Two gases A and B are at 25 o. C. Gas A has a mass of 10 grams and gas B has a mass of 20 grams. Which gas has a higher velocity (is moving faster)? GAS A 50 = ½ • 10 • X 2 X = √ 10 GAS B 50 = ½ • 20 • X 2 X = √ 5 Gas A HAS to be moving faster if the KEs are the same!

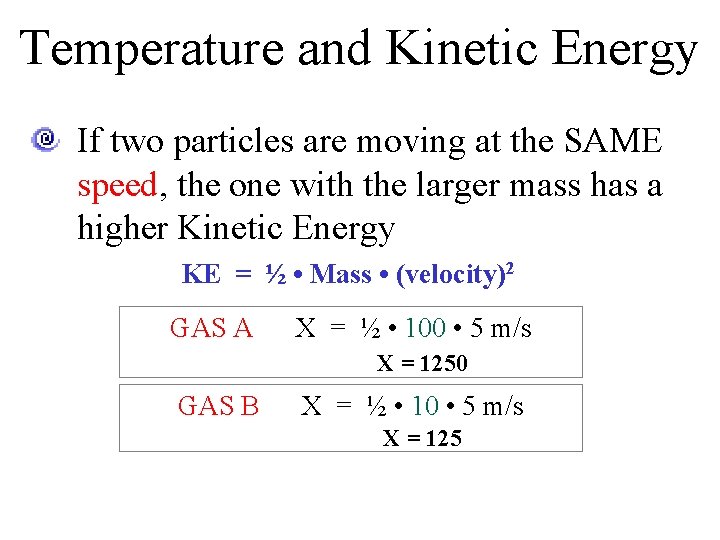
Temperature and Kinetic Energy If two particles are moving at the SAME speed, the one with the larger mass has a higher Kinetic Energy KE = ½ • Mass • (velocity)2 GAS A X = ½ • 100 • 5 m/s X = 1250 GAS B X = ½ • 10 • 5 m/s X = 125

The Nature of Gases Key parts to the Kinetic Theory of Gases: • Particles in a gas are small, hard spheres with an insignificant volume. • Motion of particles in a gas is rapid, constant, and random. • All collisions between particles in a gas are perfectly elastic. • Gas particles are NOT attracted to each other.

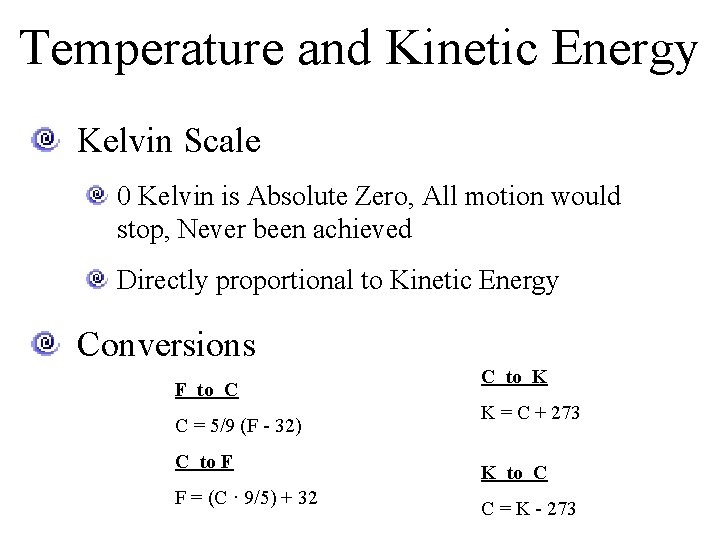
Temperature and Kinetic Energy Kelvin Scale 0 Kelvin is Absolute Zero, All motion would stop, Never been achieved Directly proportional to Kinetic Energy Conversions F to C C = 5/9 (F - 32) C to F F = (C · 9/5) + 32 C to K K = C + 273 K to C C = K - 273

Changes in Physical State Changing State 3 main states: Solid, Liquid, Gas Seven Ways to Change State Melting, Boiling, Condensing, Subliming, Evaporating, Freezing, Deposition

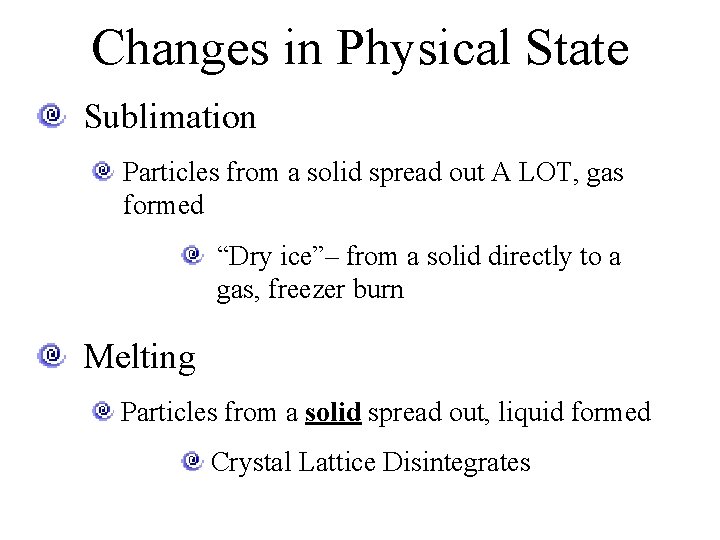
Changes in Physical State Sublimation Particles from a solid spread out A LOT, gas formed “Dry ice”– from a solid directly to a gas, freezer burn Melting Particles from a solid spread out, liquid formed Crystal Lattice Disintegrates

Changes in Physical State Freezing Particles from a liquid OR gas condense, solid formed Crystal lattice forms Boiling Particles from a liquid spread out, gas formed Molecules moving faster, very spread out Has to overcome external pressure (Atm. )

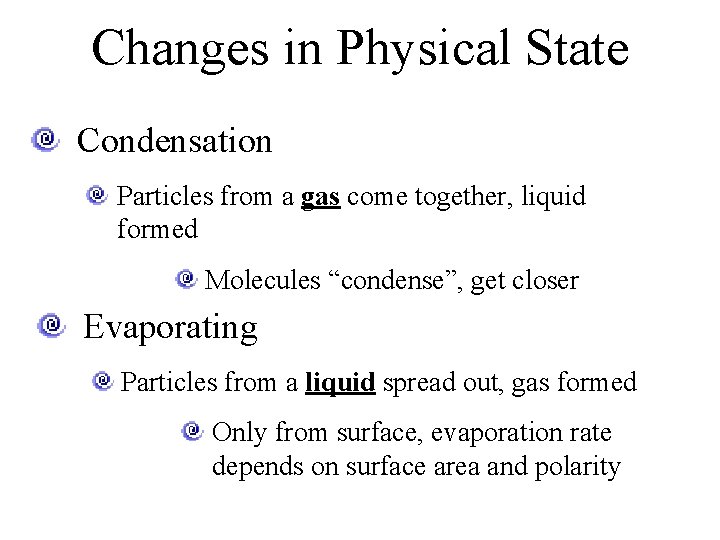
Changes in Physical State Condensation Particles from a gas come together, liquid formed Molecules “condense”, get closer Evaporating Particles from a liquid spread out, gas formed Only from surface, evaporation rate depends on surface area and polarity

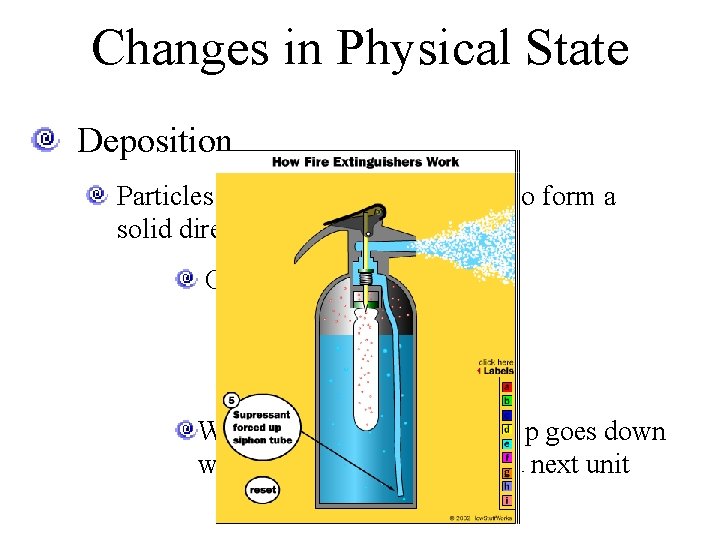
Changes in Physical State Deposition Particles from a gas come together to form a solid directly (skip liquid) CO 2 fire extinguisher We’ll talk about why the temp goes down when the pressure is released next unit

Changes in Physical State Heating and Cooling Curves Changes in temperature over time -changes in kinetic energy Changes in Slope Represent changes in state large slope = single physical state No slope (flat line) = mixture of states

Heat Transfer Heating Curves

Heat Transfer Heating Curves • Between points B and C the following is happening: • Heat is being absorbed • The temperature is NOT increasing • The solid is melting, starting at B, completely melted at C MELTING POINT is at point B

Heat Transfer Melting Point First temperature where the solid begins changes to a liquid MELTING POINT and FREEZING POINT are the same temperature melting point IF heat energy is being added (getting warmer) freezing point IF heat energy is being removed (getting colder)

Heat Transfer Heating Curves • Between points D and E the following is happening: • Heat is being absorbed • The temperature is NOT increasing • The liquid is evaporating, starting at D, completely gone at E BOILING POINT is at point D

Heat Transfer Boiling Point First temperature where liquid changes to a gas A gas will condense (phase change to liquid) at the BOILING POINT When a gas condenses it gives off energy (heat), ‘steam burns’ worse than hot water burns (more energy at point F than E)

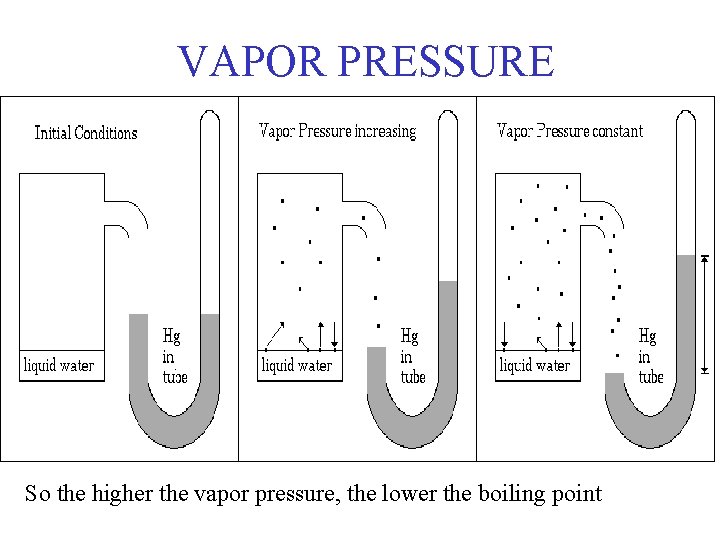
Vapor Pressure • Vapor pressure is a measure of the force exerted by a gas above a liquid. • If the rate of evaporation equals the rate of condensation, then the system is in equilibrium. • Increasing the temp, increases the vapor pressure. More of the liquid will be vaporized so more pressure.

VAPOR PRESSURE So the higher the vapor pressure, the lower the boiling point

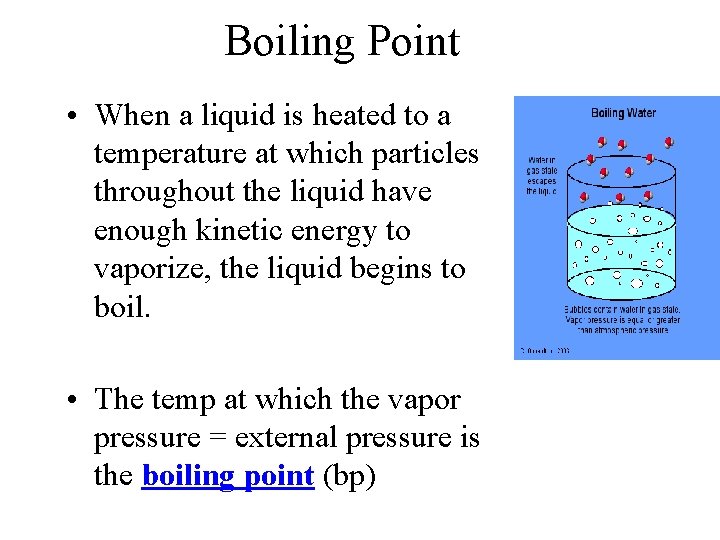
Boiling Point • When a liquid is heated to a temperature at which particles throughout the liquid have enough kinetic energy to vaporize, the liquid begins to boil. • The temp at which the vapor pressure = external pressure is the boiling point (bp)

Heat Transfer Heating Curves will look different based on several things: • The MASS of a substance • The more ‘stuff’ you have the more energy it takes to change it • The SPECIFIC HEAT of a substance • Some substances need more energy to increase in temperature

Heat Transfer Heating Curves What’s actually changing within a substance as it goes through a phase change? • From solid liquid gas, the molecules move faster and get more spread out • The attractive forces between the particles • Solids have greatest attractive force • Gases have the weakest attractive force

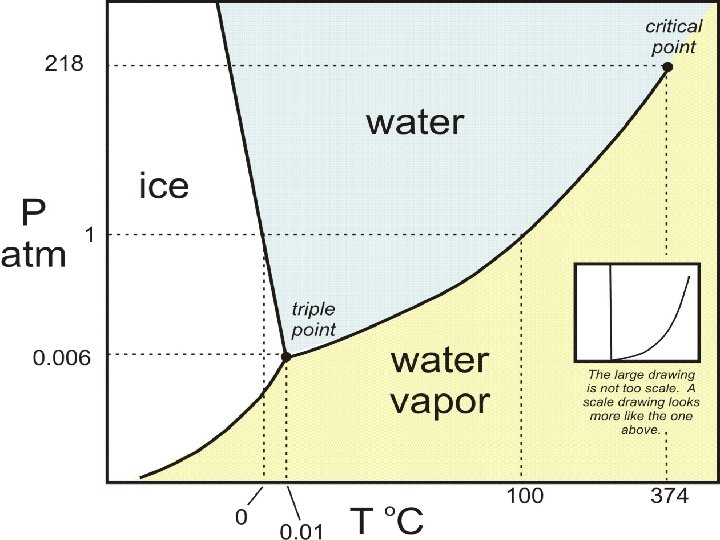
Heat Transfer Phase Diagrams Looks way different from a heating curve Shows the different phases for a substance and the relationship between pressure and temperature within those phases Triple point is the only set of conditions where all three phases exist in equilibrium


Gas Pressure

Gas Pressure is a measurement of force applied to a specific area i. e. pounds (force) per square inch (area) Gas pressure is caused by particles striking (colliding with) the walls of their container If there are no particles, then no collisions, then no pressure, which = a vacuum.

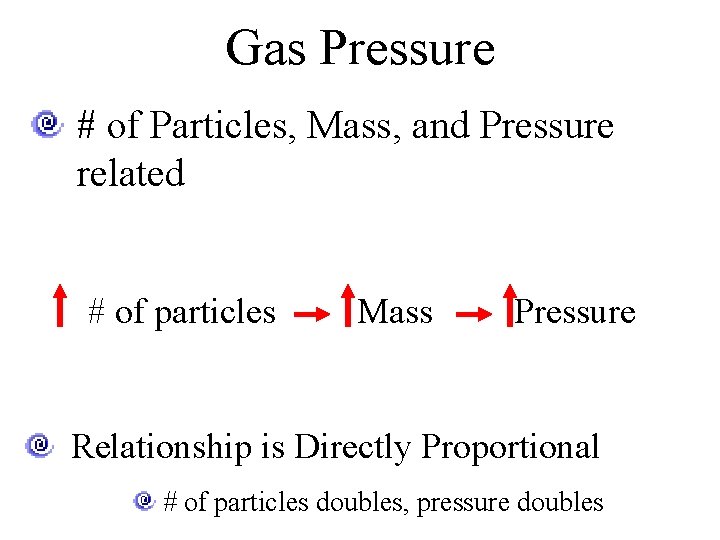
Gas Pressure # of Particles, Mass, and Pressure related # of particles Mass Pressure Relationship is Directly Proportional # of particles doubles, pressure doubles

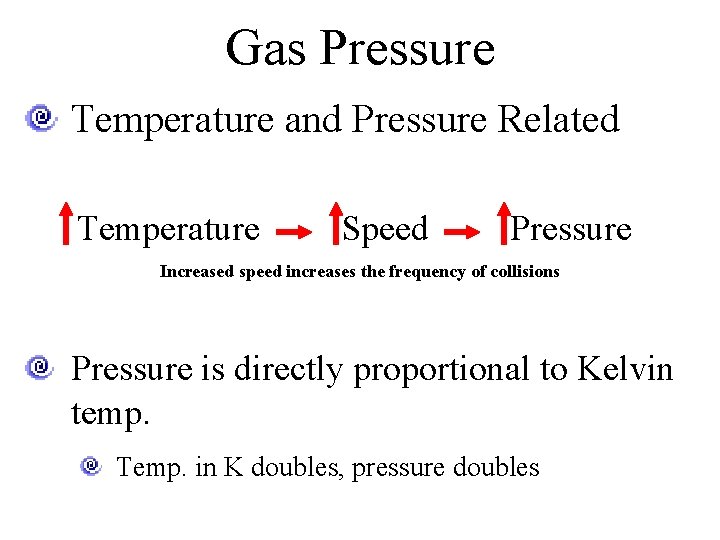
Gas Pressure Temperature and Pressure Related Temperature Speed Pressure Increased speed increases the frequency of collisions Pressure is directly proportional to Kelvin temp. Temp. in K doubles, pressure doubles

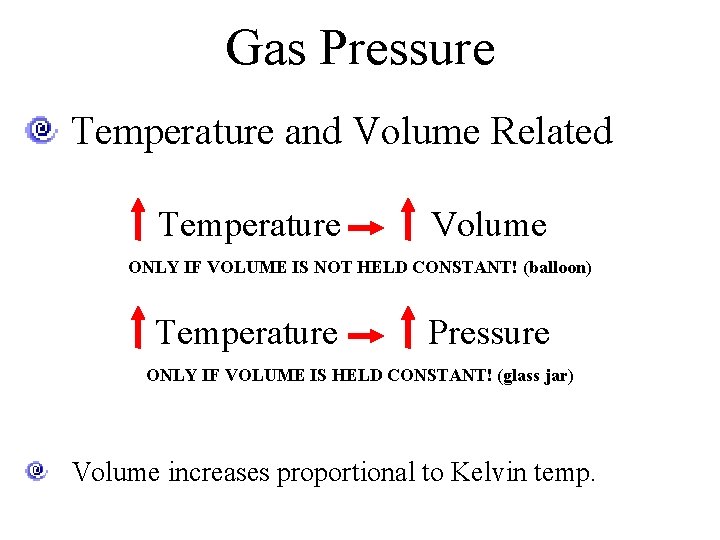
Gas Pressure Temperature and Volume Related Temperature Volume ONLY IF VOLUME IS NOT HELD CONSTANT! (balloon) Temperature Pressure ONLY IF VOLUME IS HELD CONSTANT! (glass jar) Volume increases proportional to Kelvin temp.

Gas Pressure How is Pressure Measured? Easy to compare relative pressure deflated basketball vs. inflated basketball 2 main devices to measure pressure Barometer Pressure Gauge

Gas Pressure Barometers Measure only atmospheric pressure Thank this guy Evangelista Torricelli (1608 -1647) Low Altitude = HIGH pressure High Altitude = LOW pressure

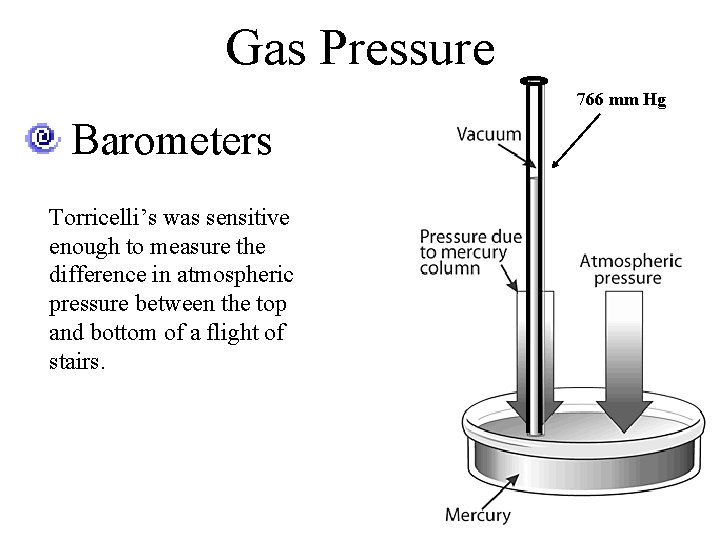
Gas Pressure 766 mm Hg Barometers Torricelli’s was sensitive enough to measure the difference in atmospheric pressure between the top and bottom of a flight of stairs.

Gas Pressure Units of Pressure 4 Main units used mm Hg Blaise Pascal 1623 -1662 Force per unit area i. e. PSI— pounds per square inch Pascals (Pa) small amount, usually use kilopascals (k. Pa) a stamp applies 1 Pa on the surface of an envelope Atmospheres (atm)

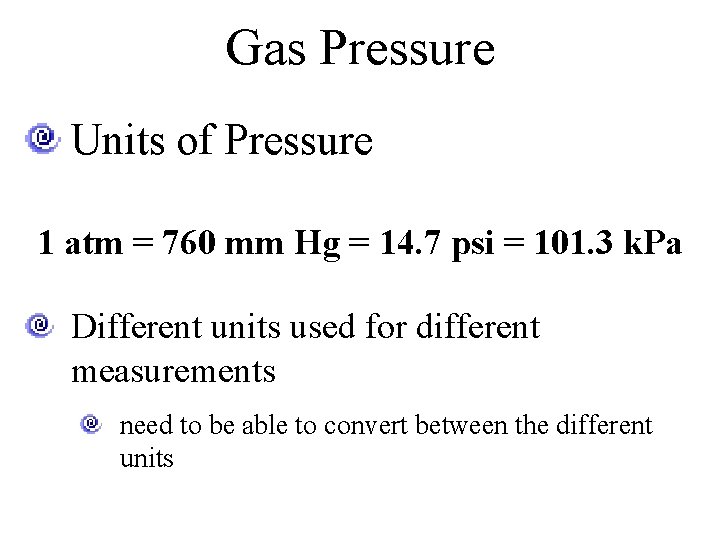
Gas Pressure Units of Pressure 1 atm = 760 mm Hg = 14. 7 psi = 101. 3 k. Pa Different units used for different measurements need to be able to convert between the different units

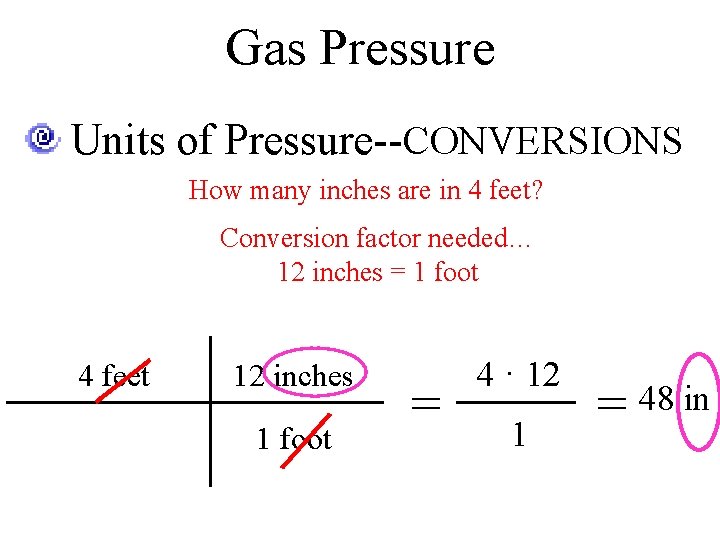
Gas Pressure Units of Pressure--CONVERSIONS Need a conversion factor Two equivalent values How many inches are in 4 feet? Conversion factor needed… 12 inches = 1 foot

Gas Pressure Units of Pressure--CONVERSIONS How many inches are in 4 feet? Conversion factor needed… 12 inches = 1 foot 4 feet 12 inches 1 foot = 4 · 12 1 = 48 in

Gas Pressure Units of Pressure--CONVERSIONS Why do conversion factors work? 1 foot = 12 inches

Gas Pressure Units of Pressure--CONVERSIONS Why do conversion factors work? 1 foot = 12 inches 1

Gas Pressure Units of Pressure--CONVERSIONS Why do conversion factors work? 1 foot 12 inches = 1 -Any number multiplied by “ 1” equals that same number - Multiplying by a conversion factor DOES not change value, ONLY changes units

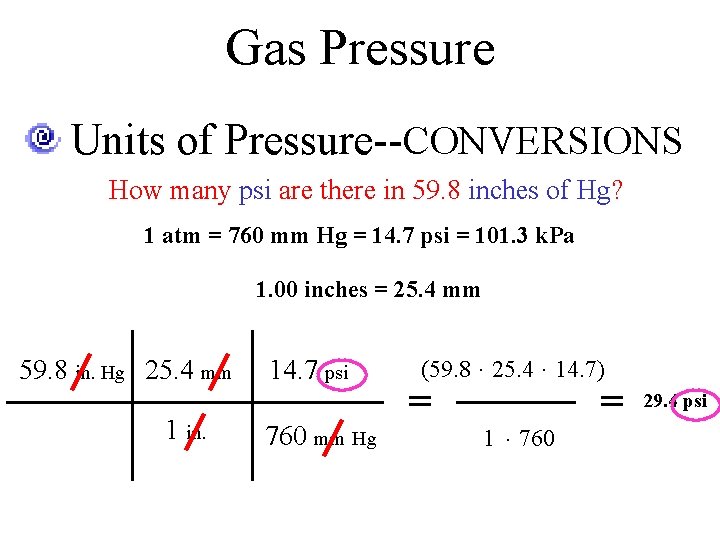
Gas Pressure Units of Pressure--CONVERSIONS How many psi are there in 59. 8 inches of Hg? 1 atm = 760 mm Hg = 14. 7 psi = 101. 3 k. Pa 1. 00 inches = 25. 4 mm 59. 8 in. Hg 25. 4 mm 1 in. 14. 7 psi 760 mm Hg (59. 8 · 25. 4 · 14. 7) = = 1 · 760 29. 4 psi