Kharkiv National Medical University Medical and bioorganic chemistry
Kharkiv National Medical University Medical and bioorganic chemistry department Lecture Fundamentals of titrimetric analysis
Outline • • 1. History of development of titrimetric analysis 2. The main concepts of titrimetric analysis 3. Equipment for titrimetric analysis 4. Requirements that apply to the standard substance 5. Requirements that apply to the reaction in titration 6. Classification of titrimetric methods 7. Types of titrimetric determinations 8. Examples of calculations in titrimetric analysis

Actuality of theme: Titrimetric (volumetric) analysis is one of the methods of quantitative analysis in analytical chemistry which is widely used in medicalbiological and sanitary-hygienic investigations for analysis of biological liquids, drinking and tap water and other objects. • This method is universal, it has sufficiently high accuracy, relatively simple and needn’t difficult measuring equipment.
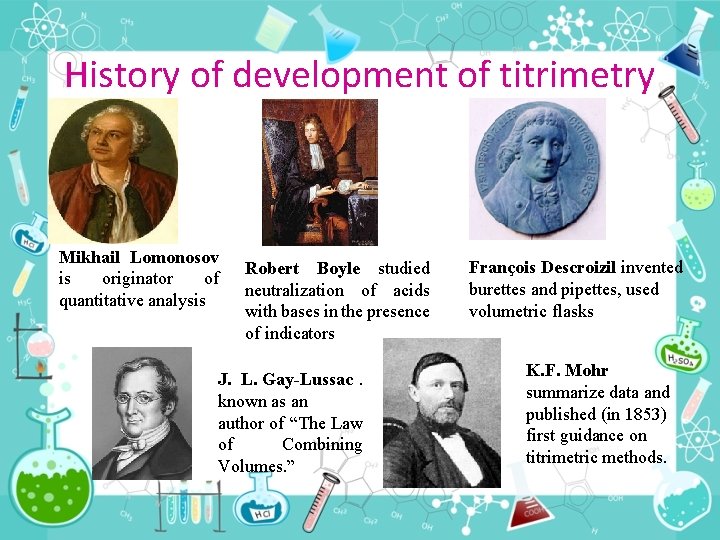
History of development of titrimetry Mikhail Lomonosov is originator of quantitative analysis Robert Boyle studied neutralization of acids with bases in the presence of indicators J. L. Gay Lussac. known as an author of “The Law of Combining Volumes. ” François Descroizil invented burettes and pipettes, used volumetric flasks K. F. Mohr summarize data and published (in 1853) first guidance on titrimetric methods.
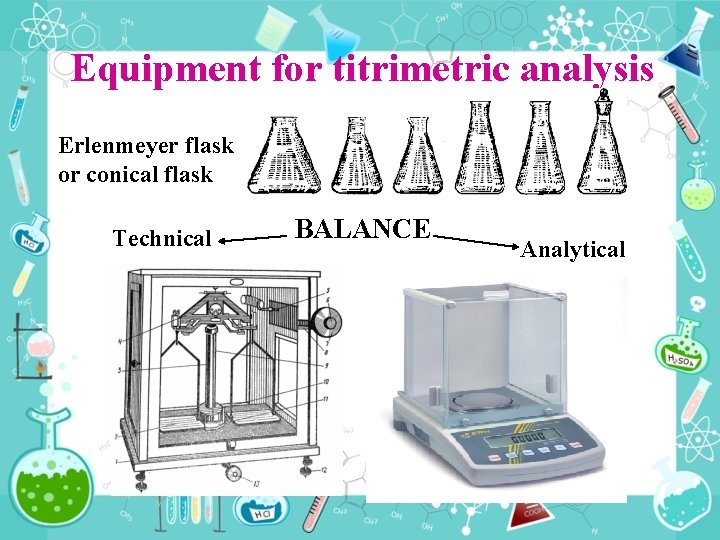
Equipment for titrimetric analysis Erlenmeyer flask or conical flask Technical BALANCE Analytical
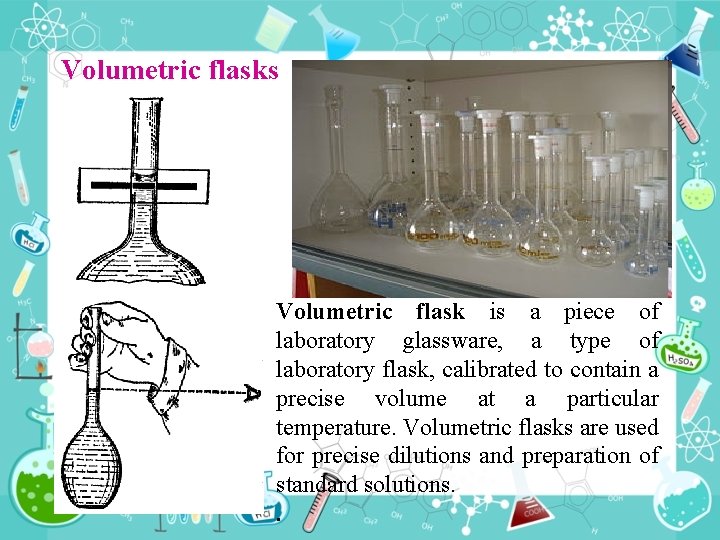
Volumetric flasks Volumetric flask is a piece of laboratory glassware, a type of laboratory flask, calibrated to contain a precise volume at a particular temperature. Volumetric flasks are used for precise dilutions and preparation of standard solutions. .
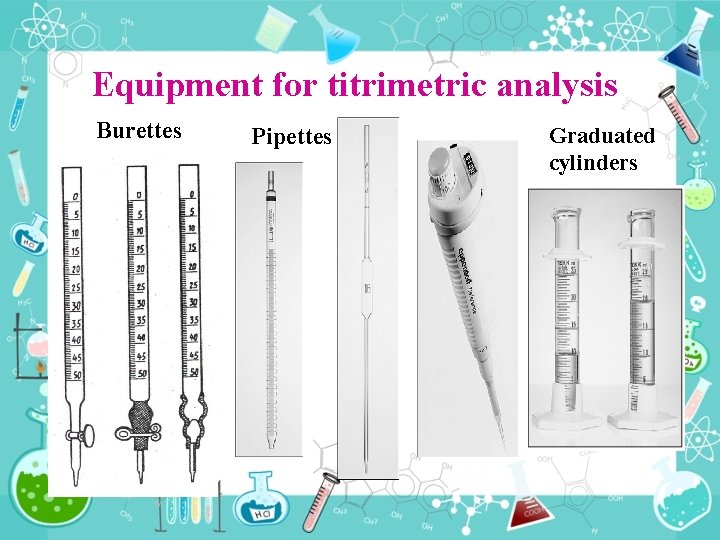
Equipment for titrimetric analysis Burettes Pipettes Graduated cylinders
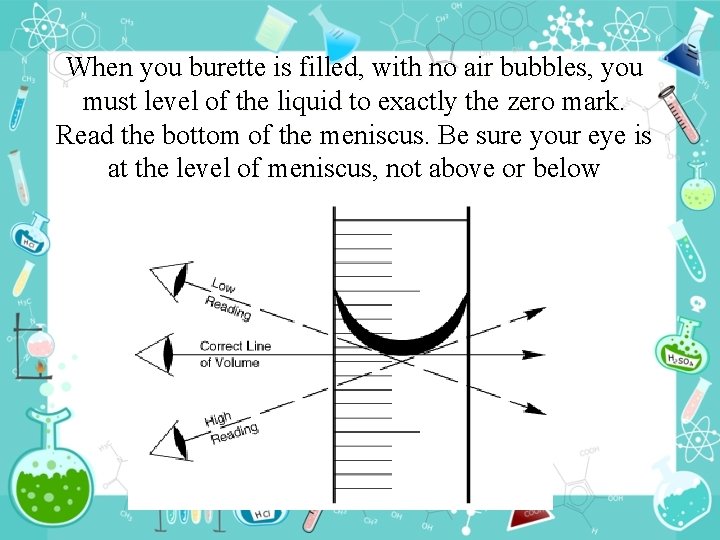
When you burette is filled, with no air bubbles, you must level of the liquid to exactly the zero mark. Read the bottom of the meniscus. Be sure your eye is at the level of meniscus, not above or below
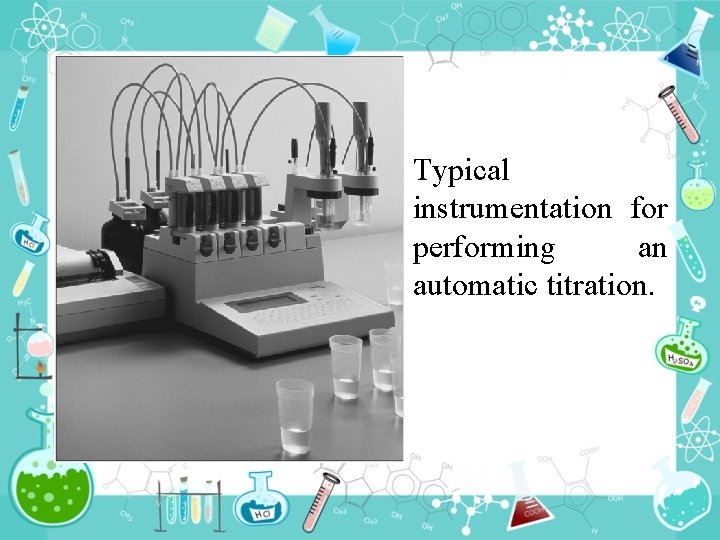
Typical instrumentation for performing an automatic titration.
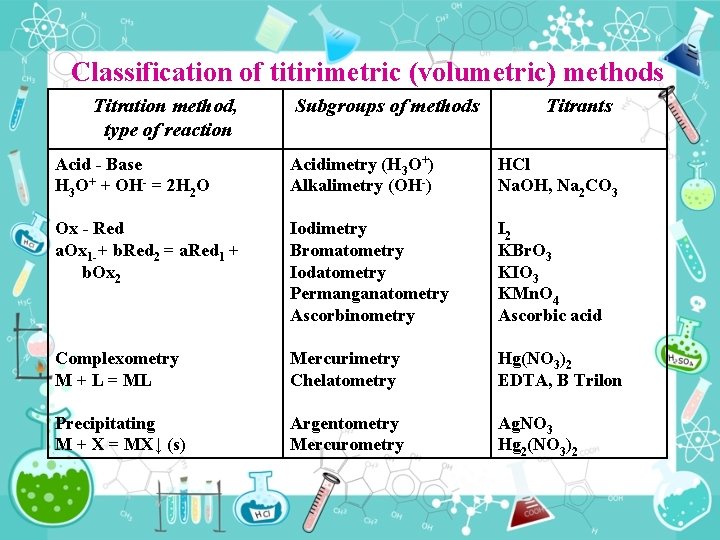
Classification of titirimetric (volumetric) methods Titration method, type of reaction Subgroups of methods Titrants Acid Base H 3 O+ + OH = 2 H 2 O Acidimetry (H 3 O+) Аlkalimetry (ОН ) HСl Na. OH, Na 2 CO 3 Ox Red а. Ox 1 + b. Red 2 = a. Red 1 + b. Ox 2 Iodimetry Bromatometry Iodatometry Permanganatometry Ascorbinometry I 2 KBr. O 3 KIO 3 KMn. O 4 Ascorbic acid Complexometry M + L = ML Мercurimetry Chelatometry Hg(NO 3)2 EDTA, B Trilon Precipitating M + X = MX↓ (s) Аrgentometry Мercurometry Ag. NO 3 Hg 2(NO 3)2
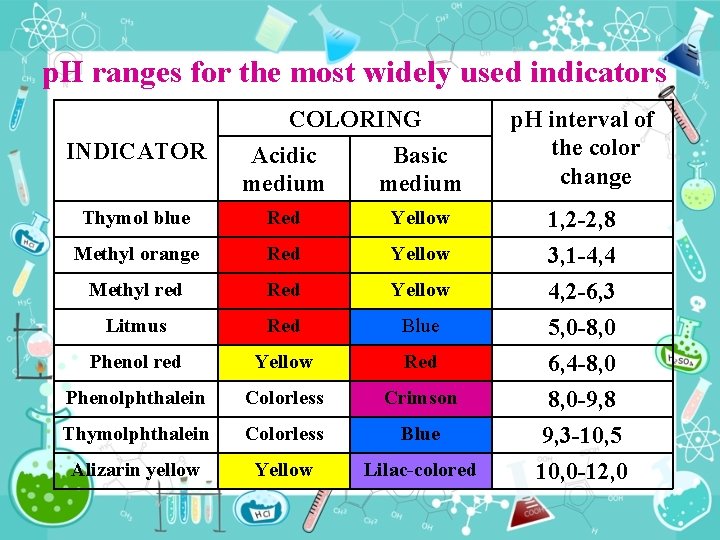
p. H ranges for the most widely used indicators INDICATOR COLORING Acidic Basic medium Thymol blue Red Yellow Methyl orange Red Yellow Methyl red Red Yellow Litmus Red Blue Phenol red Yellow Red Phenolphthalein Colorless Crimson Thymolphthalein Colorless Blue Alizarin yellow Yellow Lilac colored p. H interval of the color change 1, 2 2, 8 3, 1 4, 4 4, 2 6, 3 5, 0 8, 0 6, 4 8, 0 9, 8 9, 3 10, 5 10, 0 12, 0

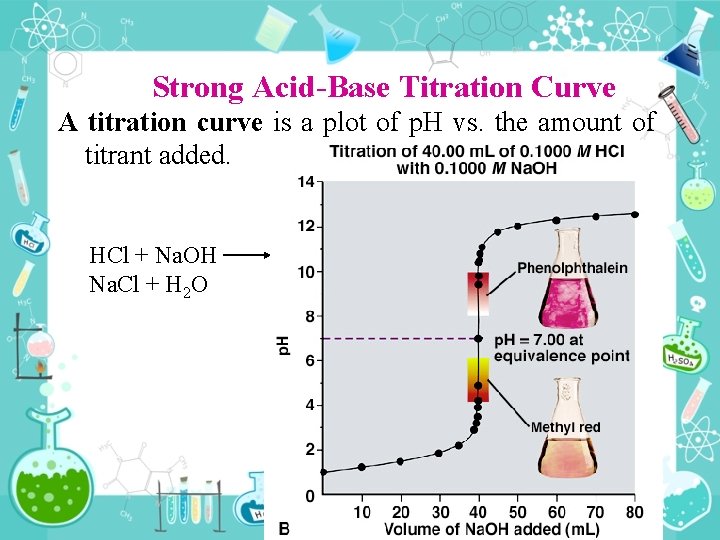
Strong Acid Base Titration Curve A titration curve is a plot of p. H vs. the amount of titrant added. HCl + Na. OH Na. Cl + H 2 O
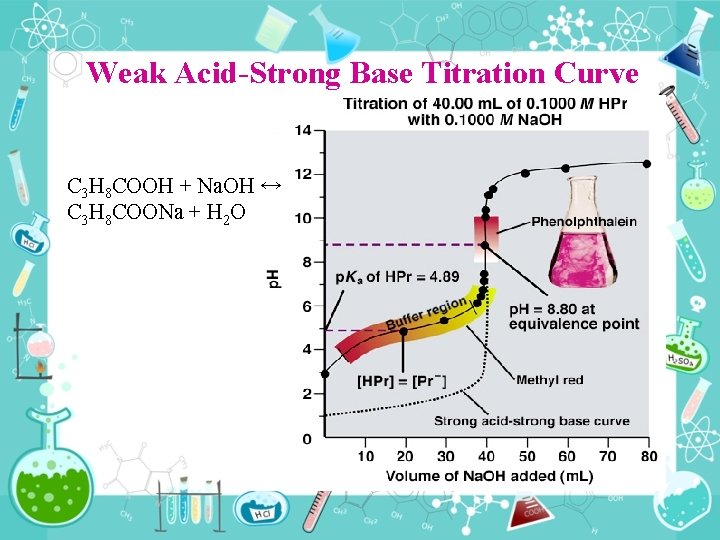
Weak Acid Strong Base Titration Curve C 3 H 8 COOH + Na. OH ↔ C 3 H 8 COONa + H 2 O
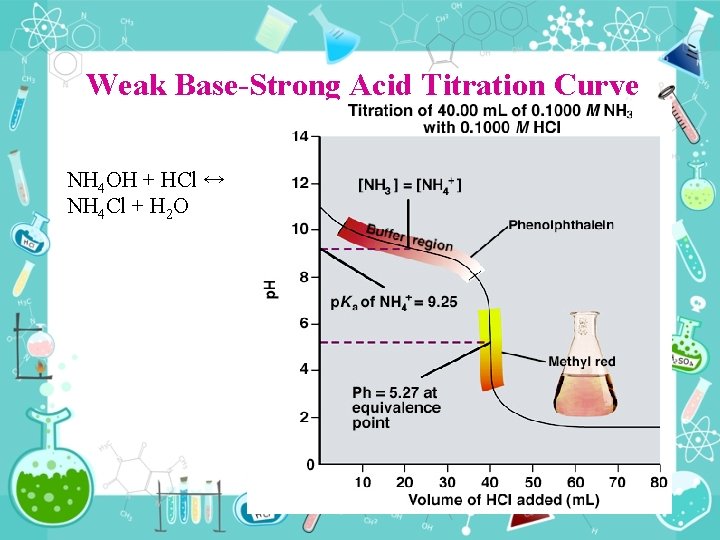
Weak Base Strong Acid Titration Curve NH 4 OH + HCl ↔ NH 4 Cl + H 2 O
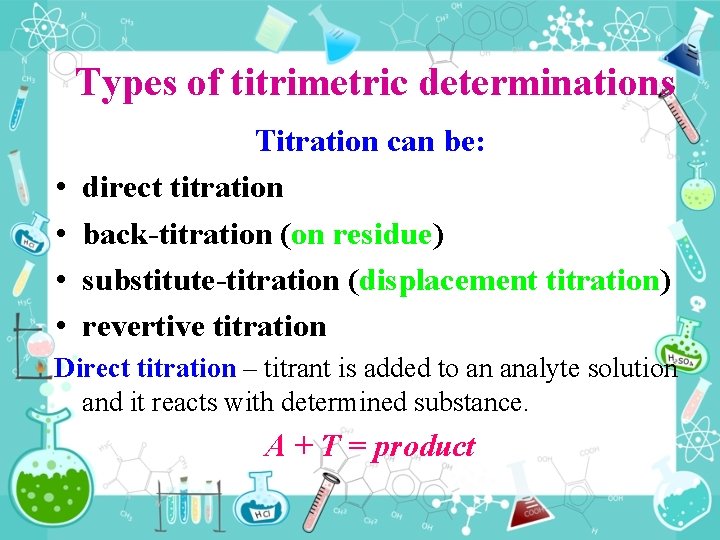
Types of titrimetric determinations • • Titration can be: direct titration back titration (on residue) substitute titration (displacement titration) revertive titration Direct titration – titrant is added to an analyte solution and it reacts with determined substance. А + Т = product
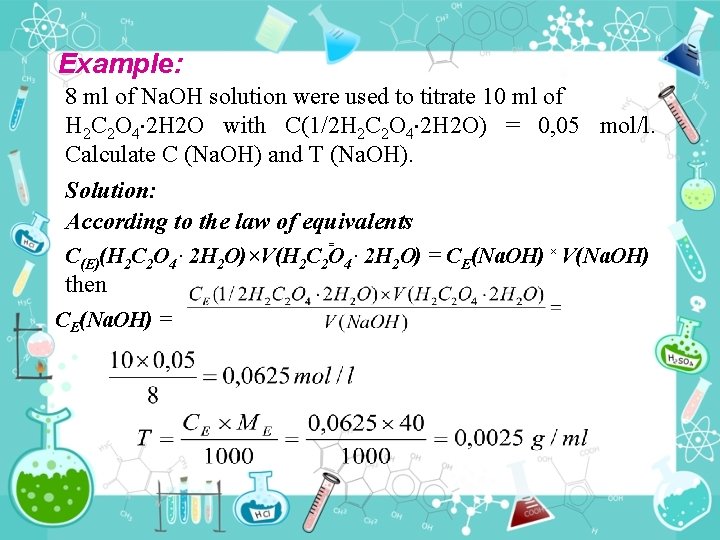
Example: 8 ml of Na. OH solution were used to titrate 10 ml of Н 2 С 2 О 4 2 Н 2 О with С(1/2 Н 2 С 2 О 4 2 Н 2 О) = 0, 05 mol/l. Calculate C (Na. OH) and T (Na. OH). Solution: According to the law of equivalents = С(E)(Н 2 С 2 О 4 2 Н 2 О)×V(Н 2 С 2 О 4. 2 Н 2 О) = CE(Na. OH) × V(Na. OH). then CE(Na. OH) =

Thank you for your attention!
- Slides: 18