Kharkiv National Medical University Department of Medical and



- Slides: 23

Kharkiv National Medical University Department of Medical and Bioorganic chemistry «Biological and Bioorganic Chemistry» Lecture № 1 CLASSIFICATION, STRUCTURE AND REACTIVITY OF BIOORGANIC COMPOUNDS Lecturer: As. Professor, Department of Medical and Bioorganic Chemistry, , Ph. D. Lukianova L. V.

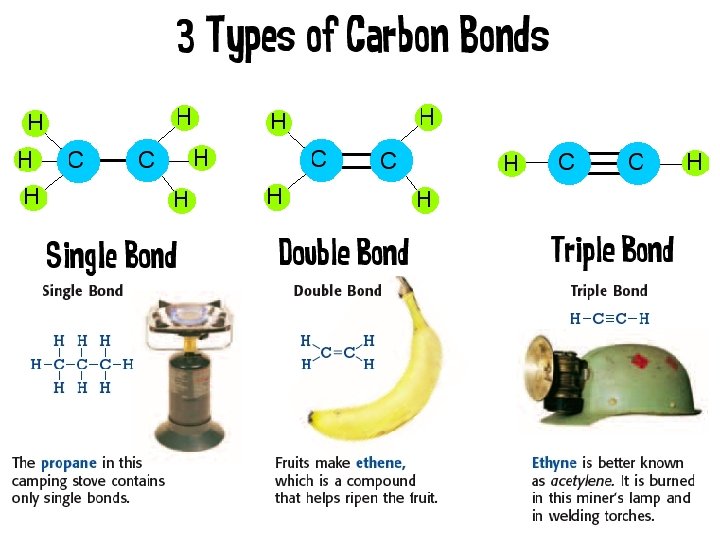
Bioorganic chemistry is based on Organic chemistry is the chemistry of the compounds of carbon. Carbon forms more compounds than any other element. 2

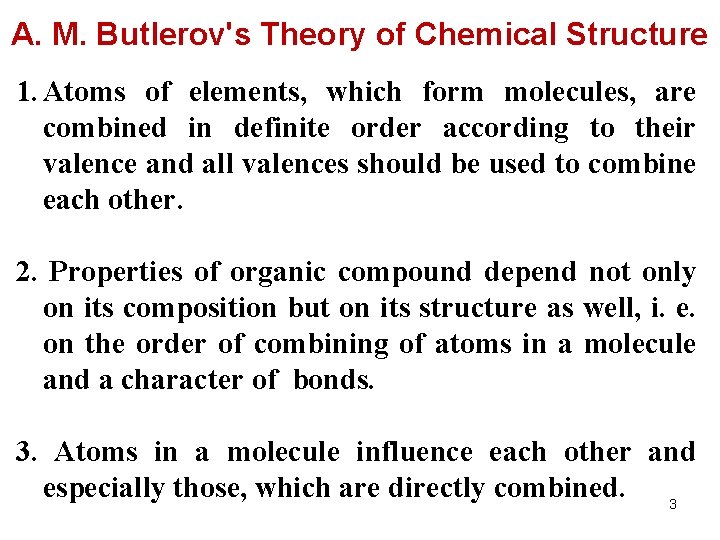
A. M. Butlerov's Theory of Chemical Structure 1. Atoms of elements, which form molecules, are combined in definite order according to their valence and all valences should be used to combine each other. 2. Properties of organic compound depend not only on its composition but on its structure as well, i. e. on the order of combining of atoms in a molecule and a character of bonds. 3. Atoms in a molecule influence each other and especially those, which are directly combined. 3

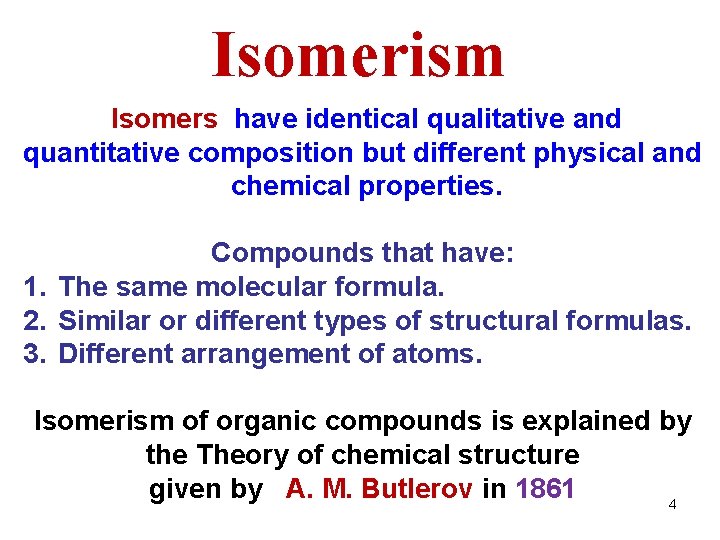
Isomerism Isomers have identical qualitative and quantitative composition but different physical and chemical properties. Compounds that have: 1. The same molecular formula. 2. Similar or different types of structural formulas. 3. Different arrangement of atoms. Isomerism of organic compounds is explained by the Theory of chemical structure given by A. M. Butlerov in 1861 4

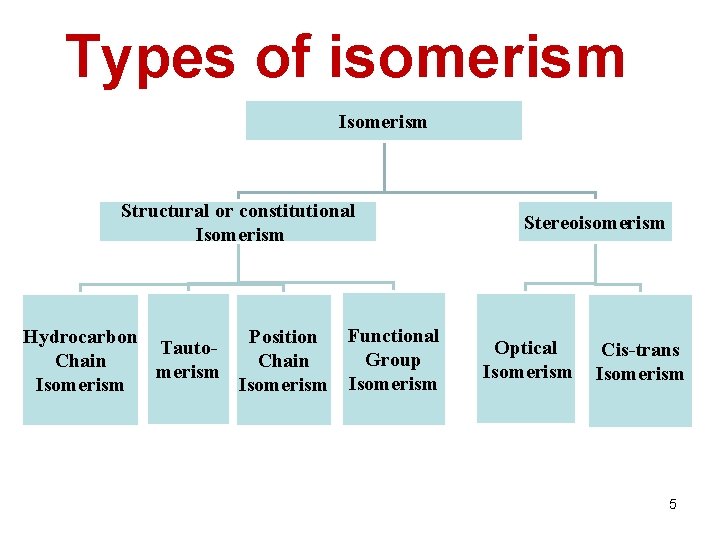
Types of isomerism Isomerism Structural or constitutional Isomerism Hydrocarbon Position Functional Tauto. Group Chain merism Isomerism Stereoisomerism Optical Isomerism Cis-trans Isomerism 5

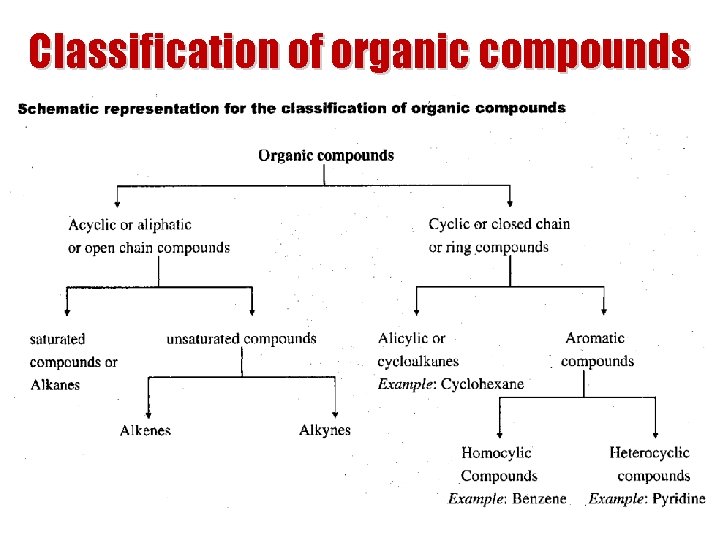
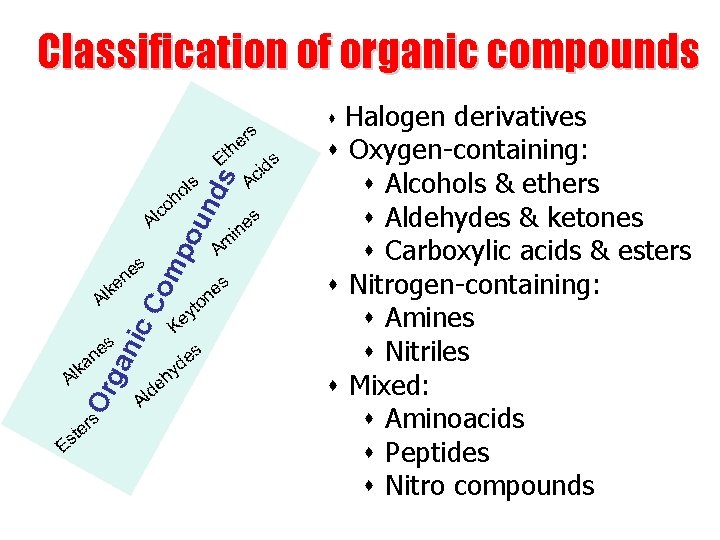
Classification of organic compounds

Classification of organic compounds Halogen derivatives s Oxygen-containing: s Alcohols & ethers s Aldehydes & ketones s Carboxylic acids & esters s Nitrogen-containing: s Amines s Nitriles s Mixed: s Aminoacids s Peptides s Nitro compounds s

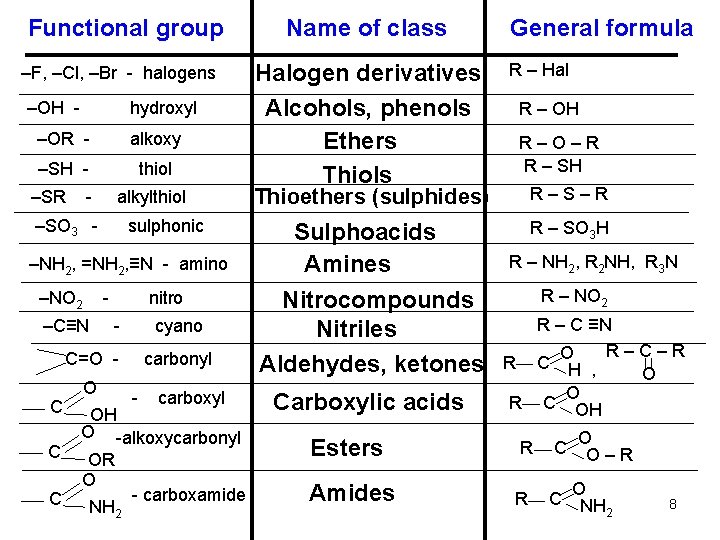
Functional group –F, –Cl, –Br - halogens –OH - hydroxyl –OR - alkoxy –SH - thiol –SR - alkylthiol –SO 3 - sulphonic –NH 2, =NH 2, ≡N - amino –NO 2 - –C≡N nitro - cyano C=O C C C O carbonyl - carboxyl OH O -alkoxycarbonyl OR O - carboxamide NH 2 Name of class Halogen derivatives Alcohols, phenols Ethers Thiols General formula R – Hal R – OH R–O–R R – SH R–S–R Thioethers (sulphides) Sulphoacids Amines Nitrocompounds Nitriles Aldehydes, ketones Carboxylic acids Esters Amides R – SO 3 H R – NH 2, R 2 NH, R 3 N R – NO 2 R – C ≡N R–C–R O R C H , O O R C OH R R C C O O–R O NH 2 8

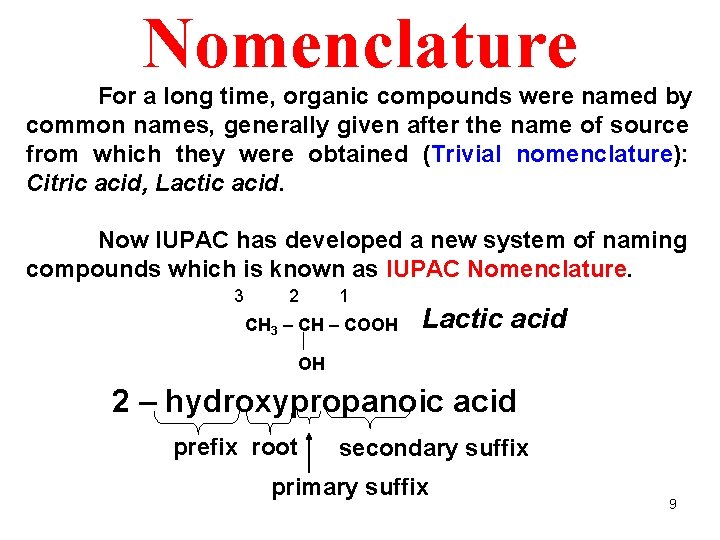
Nomenclature For a long time, organic compounds were named by common names, generally given after the name of source from which they were obtained (Trivial nomenclature): Citric acid, Lactic acid. Now IUPAC has developed a new system of naming compounds which is known as IUPAC Nomenclature. 3 2 1 CH 3 – CH – COOH Lactic acid OH 2 – hydroxypropanoic acid prefix root secondary suffix primary suffix 9

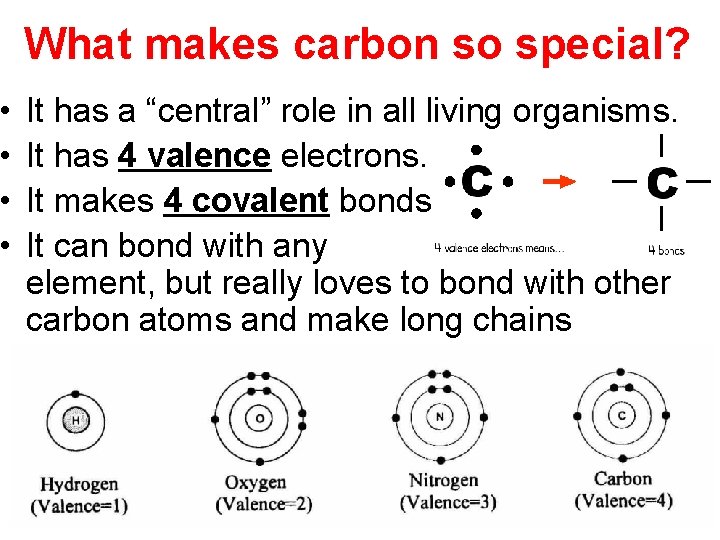
• • What makes carbon so special? It has a “central” role in all living organisms. It has 4 valence electrons. It makes 4 covalent bonds. It can bond with any element, but really loves to bond with other carbon atoms and make long chains


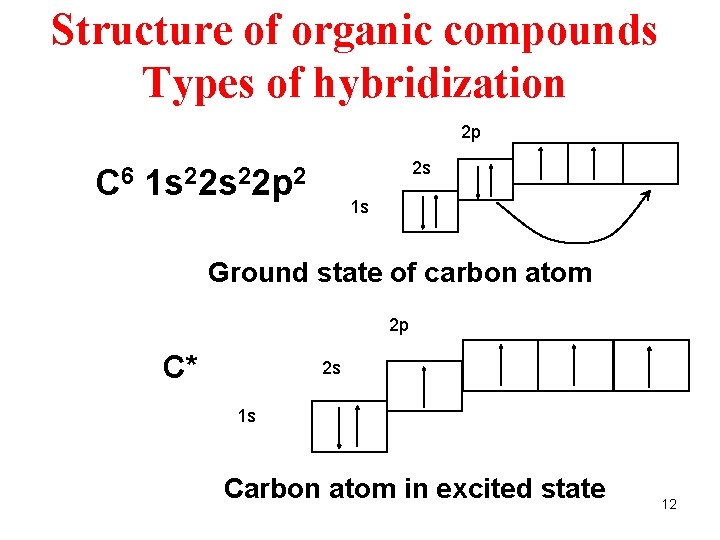
Structure of organic compounds Types of hybridization 2 p 2 s C 6 1 s 22 p 2 1 s Ground state of carbon atom 2 p C* 2 s 1 s Carbon atom in excited state 12

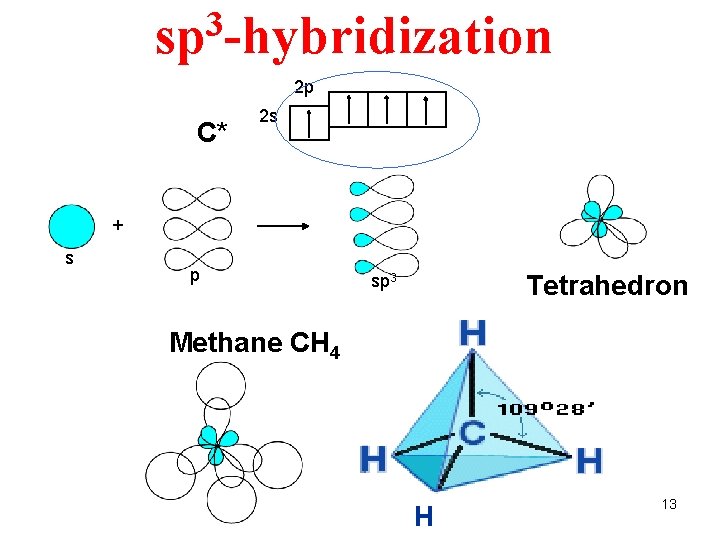
3 sp -hybridization 2 p C* 2 s + s p Tetrahedron sp 3 Methane CH 4 H 13

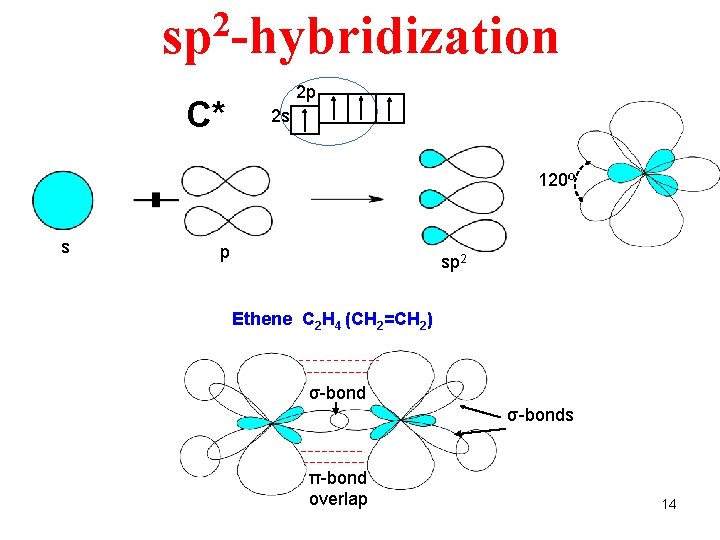
2 sp -hybridization C* 2 p 2 s 120º s p sp 2 Ethene C 2 H 4 (CH 2=CH 2) σ-bonds π-bond overlap 14

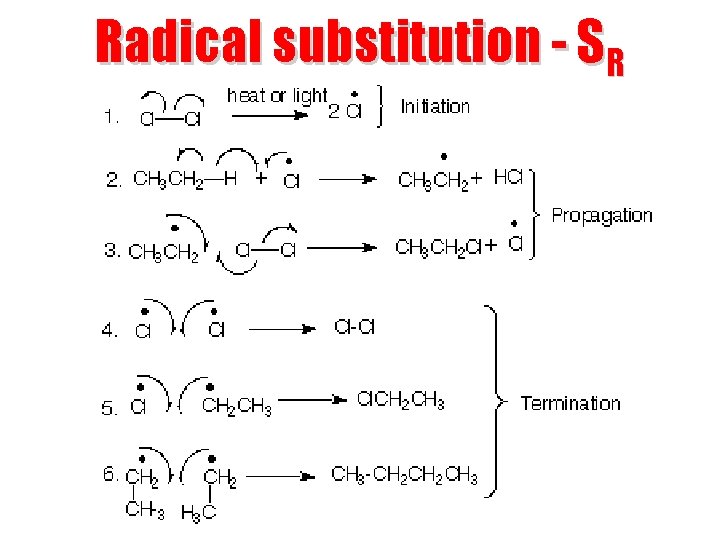
Radical substitution - SR

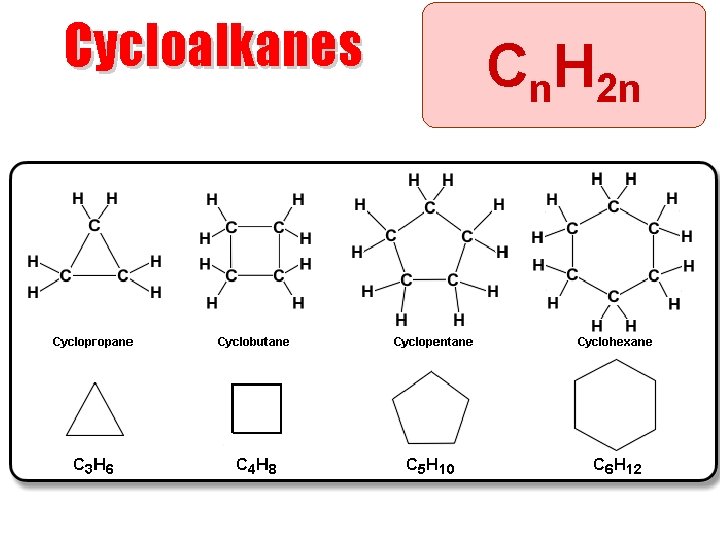
Cycloalkanes Cn. H 2 n

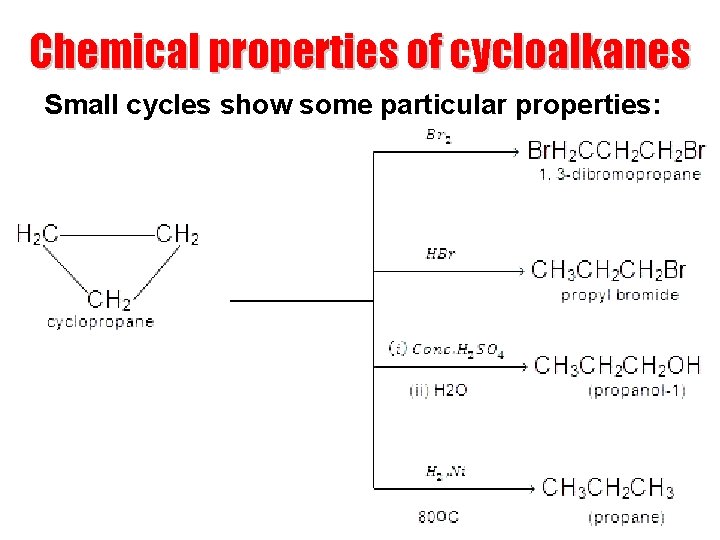
Chemical properties of cycloalkanes Small cycles show some particular properties:


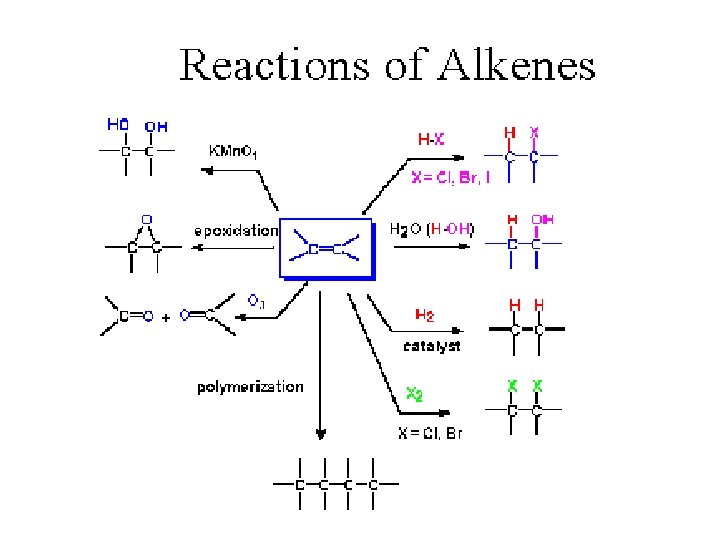
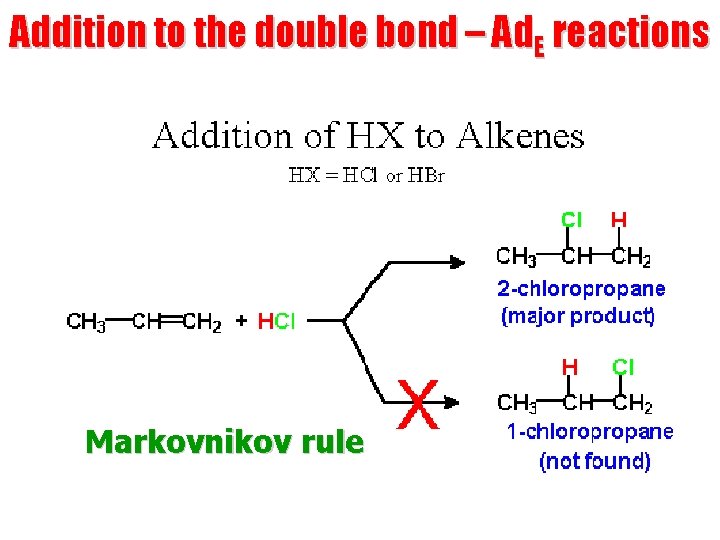
Addition to the double bond – Ad. E reactions Markovnikov rule

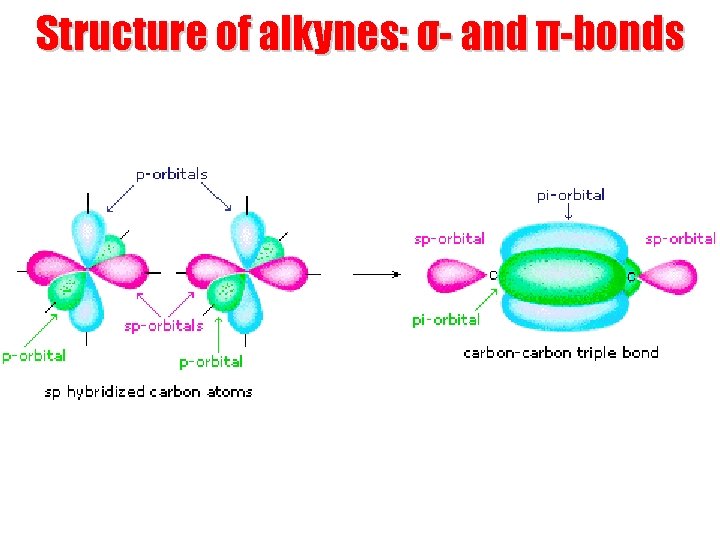
Structure of alkynes: σ- and π-bonds

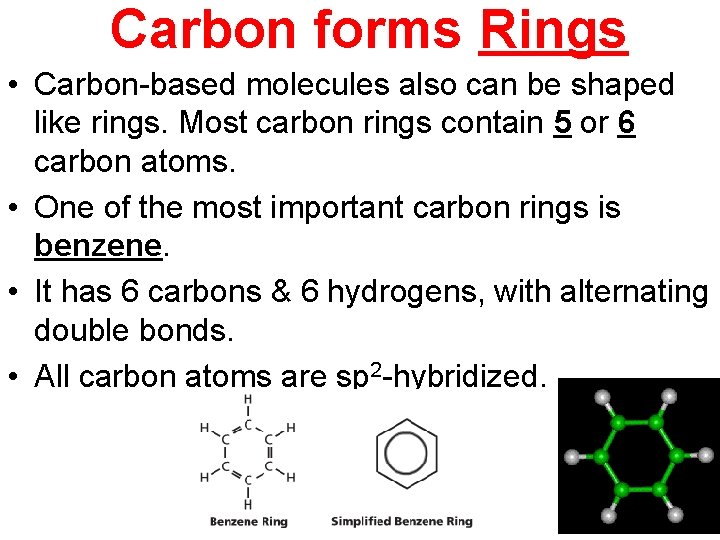
Carbon forms Rings • Carbon-based molecules also can be shaped like rings. Most carbon rings contain 5 or 6 carbon atoms. • One of the most important carbon rings is benzene. • It has 6 carbons & 6 hydrogens, with alternating double bonds. • All carbon atoms are sp 2 -hybridized.

Carbon forms Rings • Many compounds are based on Benzene. • They often have very strong smells or aromas, so they are called aromatic compounds. • An example of one aromatic compound is a molecule called vanillin.

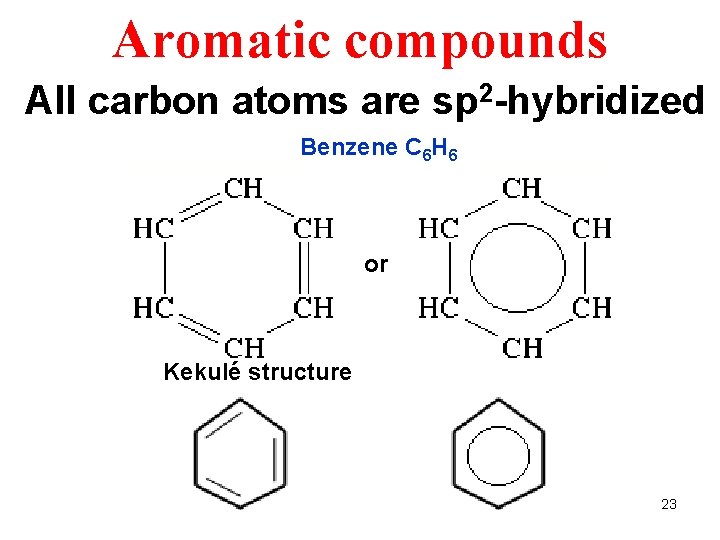
Aromatic compounds All carbon atoms are sp 2 -hybridized Benzene C 6 H 6 or Kekulé structure 23