Key Green Chemistry Research Areas A Perspective from







- Slides: 14

Key Green Chemistry Research Areas - A Perspective from Pharmaceutical Manufacturers

Membership as of August 1, 2006 ACS Green Chemistry Institute Pharmaceutical Roundtable Membership is open to all pharmaceutical research, development, and manufacturing companies. The Roundtable will be strongest when all global pharmaceutical corporations are members.

Strategic Priorities p Mission To catalyze the implementation of green chemistry and engineering in the pharmaceutical industry globally. p Strategic Priorities n Informing and Influencing the Research Agenda n Tools for Innovation n Education Resource n Global Collaboration

Inform and Influence the Research Agenda p Identify commonly used reactions in Pharmaceutical Manufacturing n n Commonly used reactions Aspirational chemical transformations p Inform research community, encourage funding agencies p Selectively fund key research areas

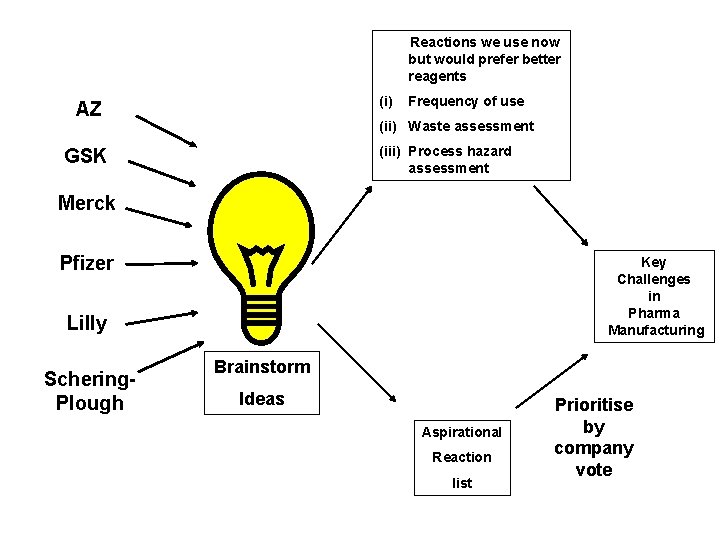
Reactions we use now but would prefer better reagents (i) AZ Frequency of use (ii) Waste assessment (iii) Process hazard assessment GSK Merck Pfizer Key Challenges in Pharma Manufacturing Lilly Schering. Plough Brainstorm Ideas Aspirational Reaction list Prioritise by company vote

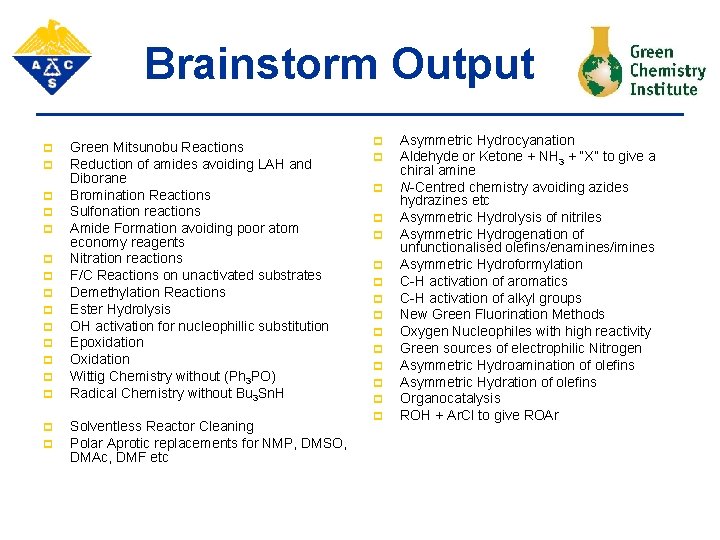
Brainstorm Output p p p p Green Mitsunobu Reactions Reduction of amides avoiding LAH and Diborane Bromination Reactions Sulfonation reactions Amide Formation avoiding poor atom economy reagents Nitration reactions F/C Reactions on unactivated substrates Demethylation Reactions Ester Hydrolysis OH activation for nucleophillic substitution Epoxidation Oxidation Wittig Chemistry without (Ph 3 PO) Radical Chemistry without Bu 3 Sn. H Solventless Reactor Cleaning Polar Aprotic replacements for NMP, DMSO, DMAc, DMF etc p p p p Asymmetric Hydrocyanation Aldehyde or Ketone + NH 3 + “X” to give a chiral amine N-Centred chemistry avoiding azides hydrazines etc Asymmetric Hydrolysis of nitriles Asymmetric Hydrogenation of unfunctionalised olefins/enamines/imines Asymmetric Hydroformylation C-H activation of aromatics C-H activation of alkyl groups New Green Fluorination Methods Oxygen Nucleophiles with high reactivity Green sources of electrophilic Nitrogen Asymmetric Hydroamination of olefins Asymmetric Hydration of olefins Organocatalysis ROH + Ar. Cl to give ROAr

Key Message Lot of commonality between the voting of the GCIrt companies

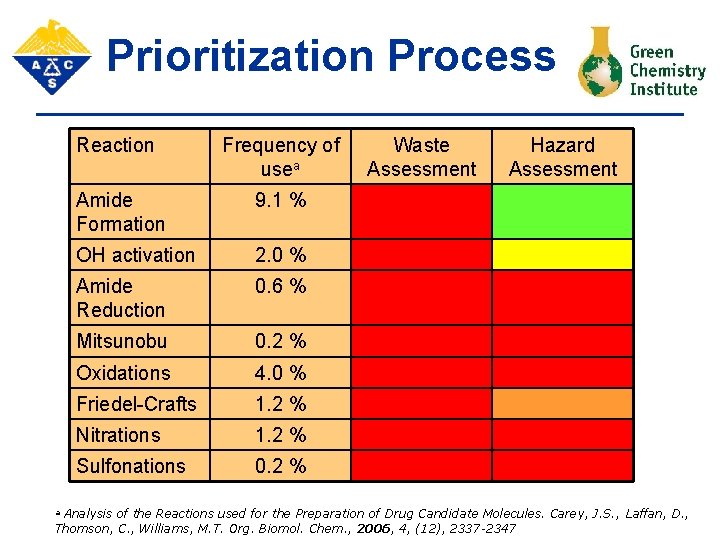
Prioritization Process Reaction Frequency of usea Amide Formation 9. 1 % OH activation 2. 0 % Amide Reduction 0. 6 % Mitsunobu 0. 2 % Oxidations 4. 0 % Friedel-Crafts 1. 2 % Nitrations 1. 2 % Sulfonations 0. 2 % Waste Assessment Hazard Assessment Analysis of the Reactions used for the Preparation of Drug Candidate Molecules. Carey, J. S. , Laffan, D. , Thomson, C. , Williams, M. T. Org. Biomol. Chem. , 2006, 4, (12), 2337 -2347 a

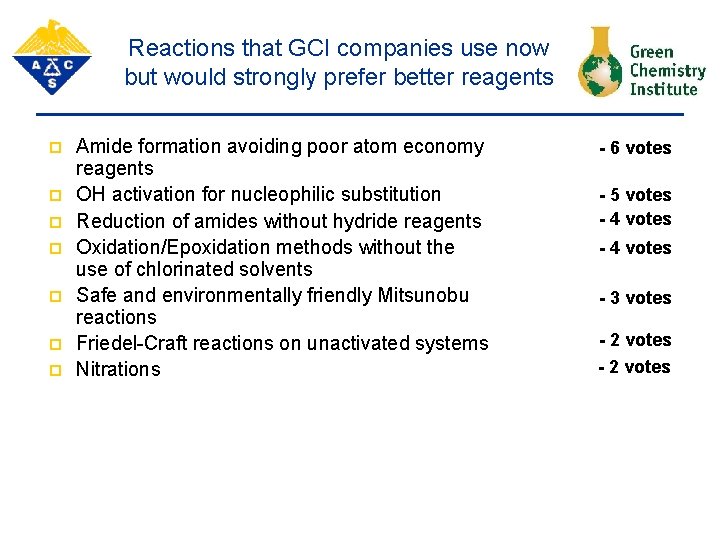
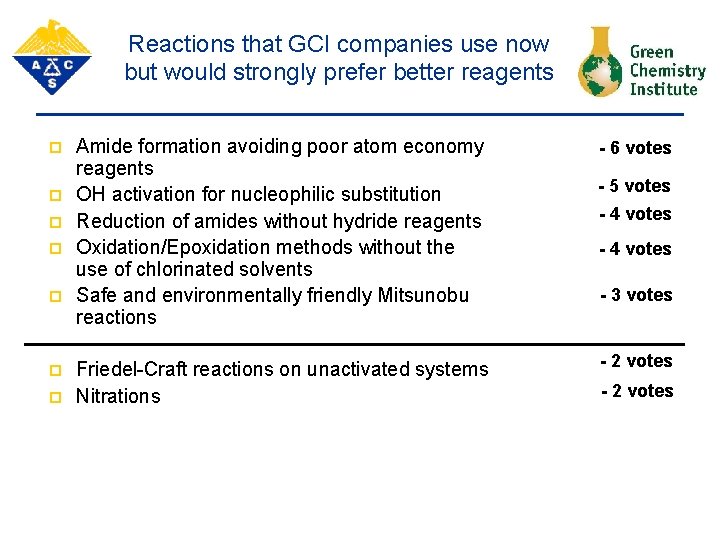
Reactions that GCI companies use now but would strongly prefer better reagents p p p p Amide formation avoiding poor atom economy reagents OH activation for nucleophilic substitution Reduction of amides without hydride reagents Oxidation/Epoxidation methods without the use of chlorinated solvents Safe and environmentally friendly Mitsunobu reactions Friedel-Craft reactions on unactivated systems Nitrations - 6 votes - 5 votes - 4 votes - 3 votes - 2 votes

Reactions that GCI companies use now but would strongly prefer better reagents p p p p Amide formation avoiding poor atom economy reagents OH activation for nucleophilic substitution Reduction of amides without hydride reagents Oxidation/Epoxidation methods without the use of chlorinated solvents Safe and environmentally friendly Mitsunobu reactions Friedel-Craft reactions on unactivated systems Nitrations - 6 votes - 5 votes - 4 votes - 3 votes - 2 votes

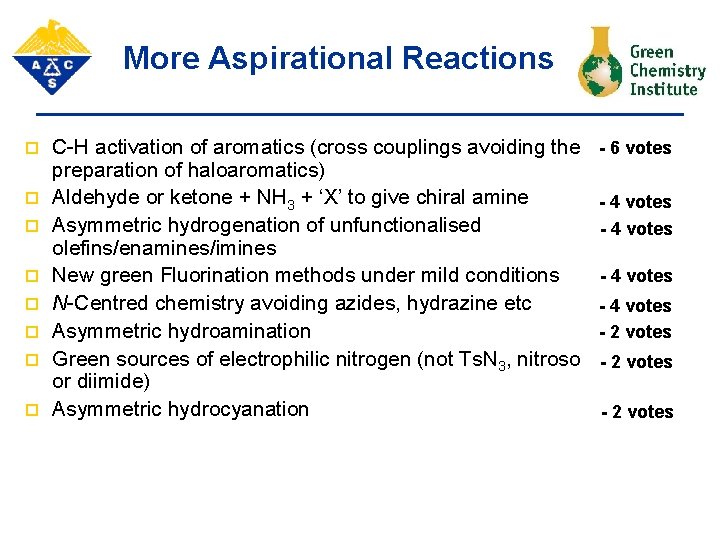
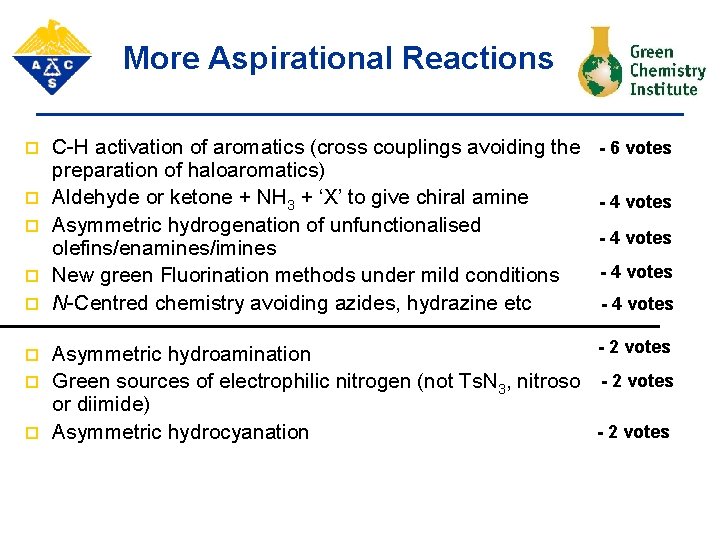
More Aspirational Reactions p p p p C-H activation of aromatics (cross couplings avoiding the preparation of haloaromatics) Aldehyde or ketone + NH 3 + ‘X’ to give chiral amine Asymmetric hydrogenation of unfunctionalised olefins/enamines/imines New green Fluorination methods under mild conditions N-Centred chemistry avoiding azides, hydrazine etc Asymmetric hydroamination Green sources of electrophilic nitrogen (not Ts. N 3, nitroso or diimide) Asymmetric hydrocyanation - 6 votes - 4 votes - 2 votes

More Aspirational Reactions p p p p C-H activation of aromatics (cross couplings avoiding the preparation of haloaromatics) Aldehyde or ketone + NH 3 + ‘X’ to give chiral amine Asymmetric hydrogenation of unfunctionalised olefins/enamines/imines New green Fluorination methods under mild conditions N-Centred chemistry avoiding azides, hydrazine etc - 6 votes - 4 votes - 2 votes Asymmetric hydroamination Green sources of electrophilic nitrogen (not Ts. N 3, nitroso - 2 votes or diimide) - 2 votes Asymmetric hydrocyanation

Key Challenges p Current Reactions n p More Aspirational Reactions n p Amide Formation, OH activation, Amide Reduction, Green Mitsunobu reactions, Oxidation/Epoxidations C-H activation or aromatics, chiral amine synthesis, Asymmetric Hydrogenation, Green Fluorination Methods, N-Centred Chemistry Key Ideas outside the Reaction theme n n Solvent less Reactor Cleaning Green alternatives to polar aprotic solvents

Questions for Discussion p p Are there anymore key research areas that did not come up in our brainstorm ? Is anyone working (or know of people working) on the key chemistry challenges ? Any good ideas to solve some of these problems ? What are the best funding opportunities ? n p How do we best tap into them ? These projects are tough and challenging. How do we entice researchers to focus their efforts on these problems ?