Key Changes to the Common Rule Regulations for























- Slides: 29

Key Changes to the Common Rule – Regulations for the Protection of Human Subjects 45 CFR 46 August 2018 Sandy Gibson Chair, IRB

Final Revisions to the Common Rule The U. S. Department of Health and Human Services and fifteen other Federal Departments and Agencies have issued final revisions to the Federal Policy for the Protections of Human Subjects (the Common Rule). The Final Rule was published in the Federal Register on January 19. 2017. It implements new steps to better protect human subjects involved in research, while facilitating valuable research and reducing burden, delay, and ambiguity for investigators. ~ HHS. gov website Final revision available at: https: //www. gpo. gov/fdsys/pkg/FR-2017 -0119/pdf/2017 -01058. pdf

Key Changes � Eliminates continuing review for most minimal risk research � Expands exemption categories and changes the review processes � Reframes informed consent information and adds required elements � Requires single IRB review of research involving external collaborators

What’s not Changing? Minimal change to IRB review of projects that involve: �More than minimal risk �Drugs/biologics/medical devices (FDA-regulated) �Collection of biospecimens �Children �Prisoners

Changes to Continuing Review �Continuing review is eliminated for studies reviewed via expedited review �The IRB can require continuing review for a study if there is cause �Also eliminated for full board projects once subject interaction is complete �Amendments and Adverse Event/ORIO reports are still required �Investigators will receive annual reminders about submitting amendments, AE/ORIOs and termination reports

Self-Determination �Researchers may be able to self-determine if their research is exempt from IRB review through the i. Medris system. �TCNJ hopes to incorporate this self-determination in the near future. �Currently, when a researcher applies for selfdeteremined exemption, there will still be IRB review to monitor and assess the accurate use of this new category. �Once review indicates accurate use of this designation, TCNJ will look towards allowing a formal, active self-determination category with auditing.

Exemption Changes

Exemption 1 – Educational Exemption What’s new? �Now must consider “adverse affects” on student learning of required educational content or on assessment of educators �Self-exemption permitted, except where research involves access to student education records under FERPA

Exemption 2 – Surveys/Interviews/Educational Tests/Public Observation ONLY What’s new? � Projects collecting sensitive and identifiable data may be exempt after “limited IRB review” (for privacy/confidentiality protections) � Clarifies that the exemption does not apply to projects involving: � � Interventions Collection of biospecimens Linking to additional personally-identifiable data Children (except for educational tests or some public observations) �Self-exemption is permitted if information is not identifiable or not sensitive

Exemption 3 – Benign Behavioral Interventions What’s new? �This exemption is completely new �Limited to research with adults What is a benign behavioral intervention? �Brief in duration �Harmless and painless �Not physically invasive �Not likely to have a significant adverse impact on subjects �Not offensive or embarrassing

Exemption 3 – Benign Behavioral Interventions �Information is collected via �Verbal or written responses (surveys/interviews) �Data entry �Observation of subject (including audiovisual recording) �Does not permit data collection via physical procedures �Physical sensors (e. g. blood pressure monitors, EEG, Fit. Bits) �Minimally invasive procedures (e. g. blood draw or saliva collection)

Exemption 3 – Benign Behavioral Interventions �Must obtain “prospective agreement to the intervention and information collection” �No deception, except where the subject is told that they will be unaware or misled about the nature or purposes of the research and they agree �Debriefing still encouraged �Self-exemption permitted for projects that do not involve deception and where information collected is not identifiable or not sensitive �“Limited IRB Review” required for projects collecting sensitive and identifiable data

Examples �Solving puzzles under various noise conditions �Playing an economic game �Being exposed to stimuli such as color, light or sound (at safe levels) �Performing cognitive tasks

Exemption 4 – Secondary Research Uses of Identifiable Private Information or Identifiable Biospecimens What’s new? � No longer limited to retrospective data review � Permits secondary use of identifiable protected health information (PHI) (with HIPAA privacy board review) � No self-exemptions

Exemptions 7 & 8 – Storage and Secondary Use of Data/Biospecimens � Related new exemptions � Exemption 7 covers the storage and maintenance of identifiable data and/or biospecimens for future research collected under broad consent (i. e. creation of a repository). More on broad consent later… � “Limited IRB review” required to assess the terms of the broad consent � Exemption 8 covers the use of data or biospecimens collected under broad consent � “Limited IRB review” required to confirm that the proposed use is consistent with the broad consent and that privacy of subjects and confidentiality of data is appropriate

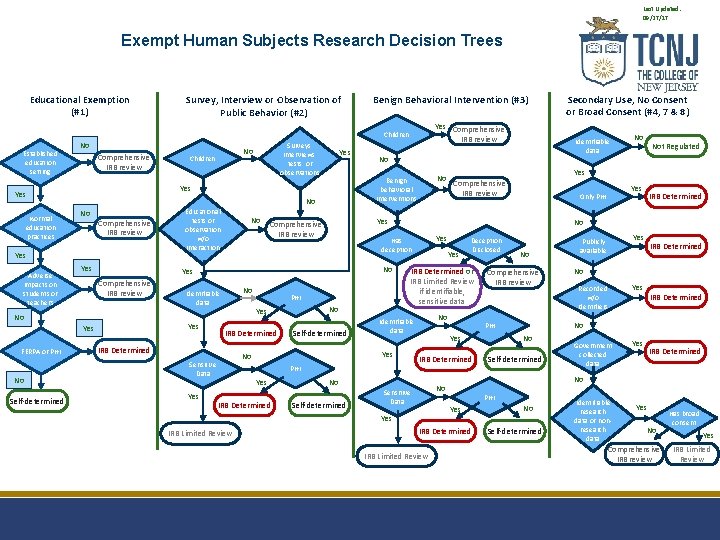
Last Updated: 09/27/17 Exempt Human Subjects Research Decision Trees Educational Exemption (#1) Survey, Interview or Observation of Public Behavior (#2) Benign Behavioral Intervention (#3) Yes Comprehensive IRB review Children Established education setting No Comprehensive IRB review Yes Self-determined No IRB Determined Self-determined IRB Determined Yes IRB Determined No Recorded w/o identifiers Yes IRB Determined No No Self-determined PHI Yes IRB Determined IRB Limited Review Yes Publicly available Government collected data Yes IRB Determined No No Sensitive Data No PHI IRB Determined Yes IRB Limited Review Yes Only PHI Comprehensive IRB review Yes No Self-determined Deception Disclosed No Identifiable data PHI Yes Not Regulated Yes Comprehensive IRB review IRB Determined or IRB Limited Review if identifiable, sensitive data Yes No Sensitive Data Yes No No No Has deception PHI Yes No Yes Comprehensive IRB review No Identifiable data Yes No No Yes Comprehensive IRB review No FERPA or PHI Educational tests or observation w/o interaction Identifiable data No Benign behavioral interventions No Yes Adverse impacts on students or teachers Children Yes Yes Normal education practices Surveys, interviews, tests, or observations No Secondary Use, No Consent or Broad Consent (#4, 7 & 8) No Self-determined Identifiable research data or nonresearch data Yes No Comprehensive IRB review Has broad consent Yes IRB Limited Review

Informed Consent Changes

Informed Consent Changes �Provide a “concise and focused presentation of key information” up front �Key information: � � Voluntary participation Summary of research procedures Risks Benefits �Brief social/behavioral consent documents may already meet this requirement �New templates will be available on the IRB website this fall

New Informed Consent Elements �New required consent element �De-identified data or biospecimens may be shared for future research (or not) �New consent elements (if applicable) �Biospecimens may be used for commercial profit (and whether the subject will share in that profit) �Clinically relevant results will be returned (or not) �Research will involve whole genome sequencing

Broad Consent for Future Research using Identified Data or Biospecimens �New provision for future storage and research use of identified data or biospecimens �Not required for storage and secondary research use of de-identified data or specimens or for uses consistent with the original informed consent � � New Exemption 7 covers the storage and maintenance of data/specimens collected with broad consent New Exemption 8 covers the secondary use data/specimens collected with broad consent

Other Consent-Related Changes �Waiver of informed consent (for secondary use of data) � Must validate why use of identified data is necessary to the research �For federally-sponsored clinical trials, a copy of the consent form must be posted on a “Federal Web site that will be established as a repository for such informed consent forms. ” OHRP defines a clinical trial as: “a research study in which one or more human subjects are prospectively assigned to one or more interventions (which may include placebo or other control) to evaluate the effect of the interventions on biomedical or behavioral healthrelated outcomes. ”

Single IRB Review Requirement �Requires that all federally-sponsored research with multi-institutional collaborators be reviewed by one designated IRB of Record �Not required until January 2020 for federally funded research �TCNJ is adopting this now for all non-federally funded research Note: NIH Single IRB (s. IRB) requirement for federally funded research is effective as of January 2019

Changes to e. Research Application � Change to i. Medris questions � Addition of Exemption Screener page � Change to Exemption detail pages �Disqualifying response moves to next question in the full review path rather than sending back to select a new application type � New Secondary Use application path for all projects involving only secondary analysis of data/specimens � Changes to Informed Consent/Child Assent selections � Changes to Waiver of Informed Consent and Waiver of Documentation criteria

Timeline for Transition � The new rule is effective January 21, 2019* for federally funded research � Federally funded research approved on or after January 19 must be compliant with new rules � Federally funded projects approved before January 19 will be approved under current rule � TCNJ has decided to implement the new rule for all non-federally funded research starting August 27, 2018.

TCNJ Common Rule Resources �Check the HRPP Common Rule & Other Changes website for updates �Important dates �Changes to institutional policies and procedures �Send questions/comments to the IRB �Check the IRB website throughout the fall �Updated IRB guidance materials and case studies �New informed consent templates and guidance �Schedule of information sessions

Federal Common Rule Resources �Federal Policy for the Protection of Human Subjects, Text of New Rule https: //www. gpo. gov/fdsys/pkg/FR-2017 -01 -19/pdf/2017 -01058. pdf �Secretary’s Advisory Committee on Human Research Protections, August 2, 2017, Letter to the HHS Secretary and Attachments, including: �Attachment B, Recommendations on Benign Behavioral Intervention �Attachment C, Recommendations for Broad Consent Guidance https: //www. hhs. gov/ohrp/sachrp-committee/recommendations/sachrprecommendations/index. html

Other NIH Policy Changes and Initiatives – NIH Clinical Trials Initiative �Intended to “enhance the accountability and transparency of clinical research” funded by the NIH � Registration of research and reporting of results on Clinical. Trials. gov (effective 1/18/2017) � Good Clinical Practice training requirement (effective 1/1/2017) � Single IRB-of-Record requirement for multisite studies (effective 1/29/2019) �Clinical trials are broadly defined and may include some basic behavioral studies that manipulate an independent variable to observe a hypothesized modification of a behavioral process �For more information, including FAQs and Case Studies, see: https: //grants. nih. gov/policy/clinical-trials. htm

Other NIH Policy Changes and Initiatives – New Certificate of Confidentiality Policy https: //grants. nih. gov/grants/guide/notice-files/NOT-OD-17 -109. html � Now a provision for Co. C as part of the terms and conditions of an NIH award � Limits disclosure “identifiable, sensitive“ information � Information/biospecimens are also considered to be identifiable if there is a very small risk of deductive disclosure � Includes all identifiable human subjects data, biospecimens, individual level human genomic data, or other research data � Effective October 1, 2017 � Applies to all NIH-funded research, beginning December 13, 2016 (part of the 21 st Century Cures Act) � NIH will continue to issue Co. Cs for other research falling under its mission

IRB Contact Information � IRB Chair � Sandy Gibson � gibsonc@tcnj. edu � 609 -771 -2136 � IRB Vice-Chair � Tamra Bireta � biretta@tcnj. edu � 609 -771 -3069 �IRB Faculty Members � School of Education � Maureen Connolly � Tabitha Dell’Angelo � Sandy Gibson � School of Science � Danielle Guarracino � Steffen Marcus � School of Humanities & Social Sciences � � � Diane Bates Tamra Bireta He. Len Chung � School of Nursing , Health & Exercise Science � Avery Faigenbaum