Key Changes to the Common Rule Regulations for

















- Slides: 30

Key Changes to the Common Rule – Regulations for the Protection of Human Subjects 45 CFR 46 http: //research-compliance. umich. edu/human-subjects/common-rule-other-changes October 2017 Cindy Shindledecker Director – IRB-HSBS

Final Revisions to the Common Rule The U. S. Department of Health and Human Services and fifteen other Federal Departments and Agencies have issued final revisions to the Federal Policy for the Protections of Human Subjects (the Common Rule). The Final Rule was published in the Federal Register on January 19. 2017. It implements new steps to better protect human subjects involved in research, while facilitating valuable research and reducing burden, delay, and ambiguity for investigators. ~ HHS. gov website Final revision available at: https: //www. gpo. gov/fdsys/pkg/FR-2017 -0119/pdf/2017 -01058. pdf

Key Changes • Eliminates continuing review for most minimal risk research • Expands exemption categories and changes the review processes • Reframes informed consent information and adds required elements • Requires single IRB review of research involving external collaborators

What’s not Changing? Minimal change to IRB review of projects that involve: • More than minimal risk • Drugs/biologics/medical devices (FDA-regulated) • Collection of biospecimens • Children • Prisoners

Changes to Continuing Review • Continuing review is eliminated for studies reviewed via expedited review • The IRB can require continuing review for a study if there is cause • Also eliminated for full board projects once subject interaction is complete • Amendments and Adverse Event/ORIO reports are still required • Investigators will receive annual reminders about submitting amendments, AE/ORIOs and termination reports

Exemption Changes

Changes to Exemption Review Processes New processes • Self-determination – smart form questions will allow the investigator to issue a self-determination letter for some exempt projects Note – a quality assurance process to validate a sample of self-determinations will be implemented • Submit to IRB – • Exemption with “limited IRB review” (new regulatory category) • For projects collecting sensitive, identifiable data, the IRB must review privacy/confidentiality protections (review an IRB member) • Standard exempt review by IRB staff member for certain types of exemptions or by investigator choice The e. Research questions will direct the application to the correct review process

Exemption 1 – Educational Exemption What’s new? • Now must consider “adverse affects” on student learning of required educational content or on assessment of educators • Self-exemption permitted, except where research involves access to student education records under FERPA

Exemption 2 – Surveys/Interviews/Educational Tests/Public Observation ONLY What’s new? • Projects collecting sensitive and identifiable data may be exempt after “limited IRB review” (for privacy/confidentiality protections) • Clarifies that the exemption does not apply to projects involving: • Interventions • Collection of biospecimens • Linking to additional personally-identifiable data • Children (except for educational tests or some public observations) • Self-exemption is permitted if information is not identifiable or not sensitive

Exemption 3 – Benign Behavioral Interventions What’s new? • This exemption is completely new – similar to Michigan Exemption 2 a but more complex! • Limited to research with adults What is a benign behavioral intervention? • • • Brief in duration Harmless and painless Not physically invasive Not likely to have a significant adverse impact on subjects Not offensive or embarrassing

Exemption 3 – Benign Behavioral Interventions • Information is collected via • Verbal or written responses (surveys/interviews) • Data entry • Observation of subject (including audiovisual recording) • Does not permit data collection via physical procedures • Physical sensors (e. g. blood pressure monitors, EEG, Fit. Bits) • Minimally invasive procedures (e. g. blood draw or saliva collection)

Exemption 3 – Benign Behavioral Interventions • Must obtain “prospective agreement to the intervention and information collection” • No deception, except where the subject is told that they will be unaware or misled about the nature or purposes of the research and they agree • Debriefing still encouraged • Self-exemption permitted for projects that do not involve deception and where information collected is not identifiable or not sensitive • “Limited IRB Review” required for projects collecting sensitive and identifiable data

Examples • Solving puzzles under various noise conditions • Playing an economic game • Being exposed to stimuli such as color, light or sound (at safe levels) • Performing cognitive tasks

Exemption 4 – Secondary Research Uses of Identifiable Private Information or Identifiable Biospecimens What’s new? • No longer limited to retrospective data review • Permits secondary use of identifiable protected health information (PHI) (with HIPAA privacy board review) • No self-exemptions

Exemptions 7 & 8 – Storage and Secondary Use of Data/Biospecimens • Related new exemptions • Exemption 7 covers the storage and maintenance of identifiable data and/or biospecimens for future research collected under broad consent (i. e. creation of a repository). More on broad consent later… • “Limited IRB review” required to assess the terms of the broad consent • Exemption 8 covers the use of data or biospecimens collected under broad consent • “Limited IRB review” required to confirm that the proposed use is consistent with the broad consent and that privacy of subjects and confidentiality of data is appropriate

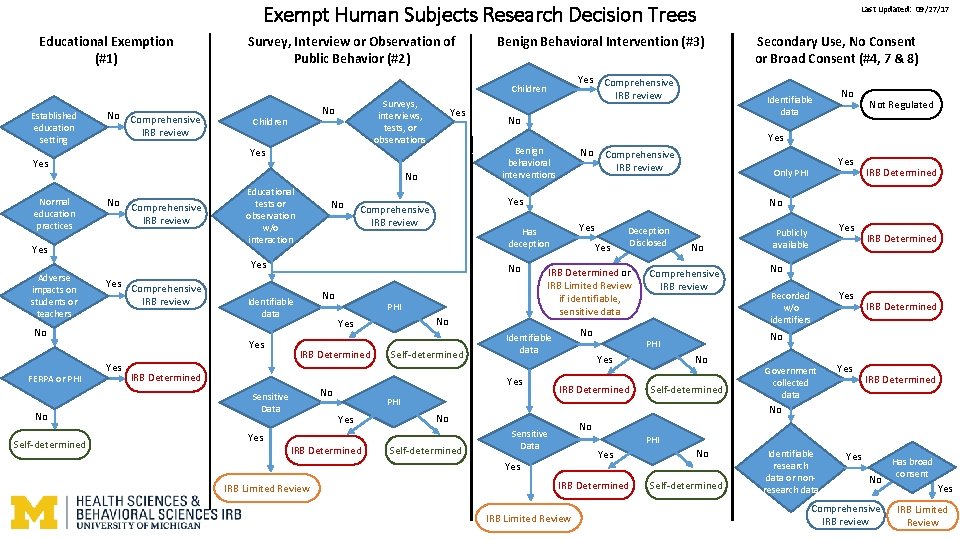
Exempt Human Subjects Research Decision Trees Educational Exemption (#1) Survey, Interview or Observation of Public Behavior (#2) Benign Behavioral Intervention (#3) Yes Comprehensive IRB review Children Established education setting No Comprehensive IRB review No Children Yes Comprehensive IRB review Yes Educational tests or observation w/o interaction No Yes Comprehensive IRB review No FERPA or PHI No Self-determined Yes No PHI No Yes IRB Determined Self-determined IRB Determined Sensitive Data Yes IRB Determined Yes No IRB Determined Yes Publicly available IRB Determined No Recorded w/o identifiers Yes IRB Determined No PHI IRB Determined No Self-determined Government collected data Yes IRB Determined No No Sensitive Data PHI Yes IRB Limited Review Yes Only PHI Comprehensive IRB review Yes No Self-determined Deception Disclosed No Identifiable data PHI Yes Comprehensive IRB review IRB Determined or IRB Limited Review if identifiable, sensitive data Yes No Not Regulated No Has deception No Identifiable data No Yes Comprehensive IRB review Yes Adverse impacts on students or teachers No Yes No No Secondary Use, No Consent or Broad Consent (#4, 7 & 8) Identifiable data No Benign behavioral interventions Yes Normal education practices Surveys, interviews, tests, or observations Last Updated: 09/27/17 IRB Determined IRB Limited Review No Self-determined Identifiable research data or nonresearch data Yes No Comprehensive IRB review Has broad consent Yes IRB Limited Review

Informed Consent Changes

Informed Consent Changes • Provide a “concise and focused presentation of key information” up front • Key information: • • Voluntary participation Summary of research procedures Risks Benefits • Brief social/behavioral consent documents may already meet this requirement • New templates will be available on the IRB-HSBS website this fall

New Informed Consent Elements • New required consent element • De-identified data or biospecimens may be shared for future research (or not) • New consent elements (if applicable) • Biospecimens may be used for commercial profit (and whether the subject will share in that profit) • Clinically relevant results will be returned (or not) • Research will involve whole genome sequencing

Broad Consent for Future Research using Identified Data or Biospecimens • New provision to for future storage and research use of identified data or biospecimens • Not required for storage and secondary research use of de-identified data or specimens or for uses consistent with the original informed consent • New Exemption 7 covers the storage and maintenance of data/specimens collected with broad consent • New Exemption 8 covers the secondary use data/specimens collected with broad consent

Other Consent-Related Changes • Waiver of informed consent (for secondary use of data) • Must validate why use of identified data is necessary to the research • For federally-sponsored clinical trials, a copy of the consent form must be posted on a “Federal Web site that will be established as a repository for such informed consent forms. ” OHRP defines a clinical trial as: “a research study in which one or more human subjects are prospectively assigned to one or more interventions (which may include placebo or other control) to evaluate the effect of the interventions on biomedical or behavioral healthrelated outcomes. ”

Single IRB Review Requirement • Requires that all federally-sponsored research with multi-institutional collaborators be reviewed by one designated IRB of Record • Not required until January 2020 Note: NIH Single IRB (s. IRB) requirement is effective as of January 25, 2018

Changes to e. Research Application • Change to Application Type page • Addition of Exemption Screener page • Change to Exemption detail pages • Disqualifying response moves to next question in the full review path rather than sending back to select a new application type • New Secondary Use application path for all projects involving only secondary analysis of data/specimens • Changes to Informed Consent/Child Assent selections • Changes to Waiver of Informed Consent and Waiver of Documentation criteria

Timeline for Transition • The new rule is effective January 19, 2018* • Projects approved on or after January 19 must be compliant with new rules • Projects approved before January 19 will be approved under current rule • Updates regarding the transition will be provided to the community throughout the fall *Note: Implementation date for some aspects of rule may be delayed

U-M Common Rule Resources • Check the HRPP Common Rule & Other Changes website for updates http: //research-compliance. umich. edu/human-subjects/common-rule-other-changes • Important dates • Changes to institutional policies and procedures • Send questions/comments to the IRBs/HRPP via our survey tool • Check the IRB-HSBS website throughout the fall http: //research-compliance. umich. edu/irb-health-sciences-and-behavioral-sciences-hsbs • Updated IRB-HSBS guidance materials and case studies • New informed consent templates and guidance • Schedule of information sessions

Federal Common Rule Resources • Federal Policy for the Protection of Human Subjects, Text of New Rule https: //www. gpo. gov/fdsys/pkg/FR-2017 -01 -19/pdf/2017 -01058. pdf • Secretary’s Advisory Committee on Human Research Protections, August 2, 2017, Letter to the HHS Secretary and Attachments, including: • Attachment B, Recommendations on Benign Behavioral Intervention • Attachment C, Recommendations for Broad Consent Guidance https: //www. hhs. gov/ohrp/sachrp-committee/recommendations/sachrprecommendations/index. html

Other NIH Policy Changes and Initiatives – NIH Clinical Trials Initiative • Intended to “enhance the accountability and transparency of clinical research” funded by the NIH • Registration of research and reporting of results on Clinical. Trials. gov (effective 1/18/2017) • Good Clinical Practice training requirement (effective 1/1/2017) • Single IRB-of-Record requirement for multisite studies (effective 1/25/2018) • Clinical trials are broadly defined and may include some basic behavioral studies that manipulate an independent variable to observe a hypothesized modification of a behavioral process • For more information, including FAQs and Case Studies, see: https: //grants. nih. gov/policy/clinical-trials. htm

Other NIH Policy Changes and Initiatives – New Certificate of Confidentiality Policy https: //grants. nih. gov/grants/guide/notice-files/NOT-OD-17 -109. html • Now a provision for Co. C as part of the terms and conditions of an NIH award • Limits disclosure “identifiable, sensitive“ information • Information/biospecimens are also considered to be identifiable if there is a very small risk of deductive disclosure • Includes all identifiable human subjects data, biospecimens, individual level human genomic data, or other research data • Effective October 1, 2017 • Applies to all NIH-funded research, beginning December 13, 2016 (part of the 21 st Century Cures Act) • NIH will continue to issue Co. Cs for other research falling under its mission

IRB-HSBS Contact Information IRB-HSBS office irbhsbs@umich. edu 734 -936 -0933 Cindy Shindledecker, Director, IRB-HSBS cshindle@umich. edu 734 -615 -9466

Questions?