Ken Rogers Miami Killian An acidbase titration is

Ken Rogers Miami Killian

An acid-base titration is a laboratory procedure where you figure out the concentration of a base solution by reacting it with a known concentration of an acid solution. Or you figure out the concentration of the acid from a known concentration of base.

The reaction is a neutralization reaction because the acid and base will react to produce water (and a salt). HCl + Na. OH a Na. Cl + H 2 O The reaction is double replacement. HCl + Na. OH a + A simulation of the titration lab follows, and then Na. OH HCl we’ll go over how to calculate the concentration from the results you get in the titration.
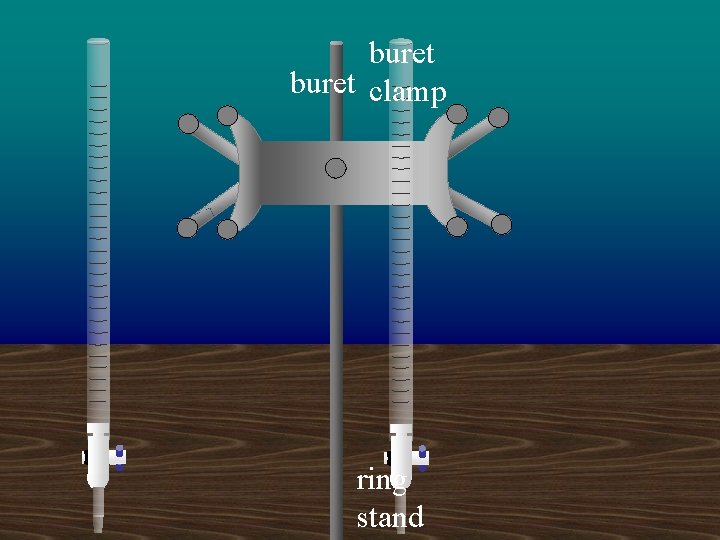
buret clamp ring stand
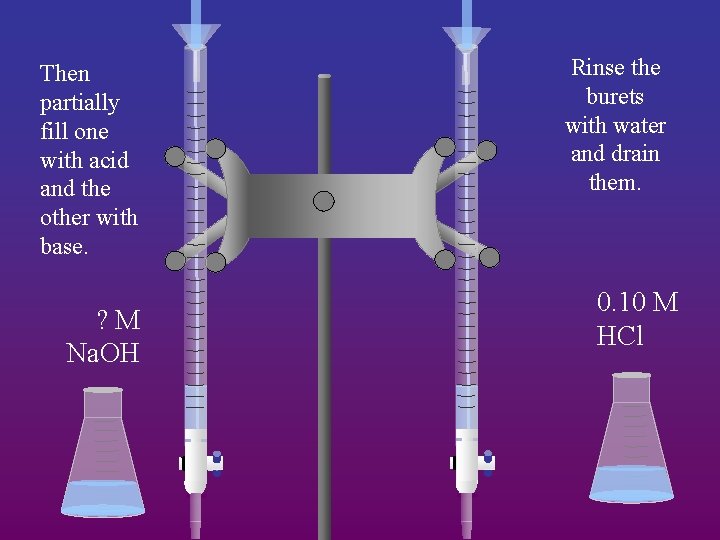
Then partially fill one with acid and the other with base. ? M Na. OH Rinse the burets with water and drain them. 0. 10 M HCl

If the buret had water in it, it would dilute the solution. So you rinse the buret with the solution before you fill it up.
? M Na. OH 0. 10 M HCl
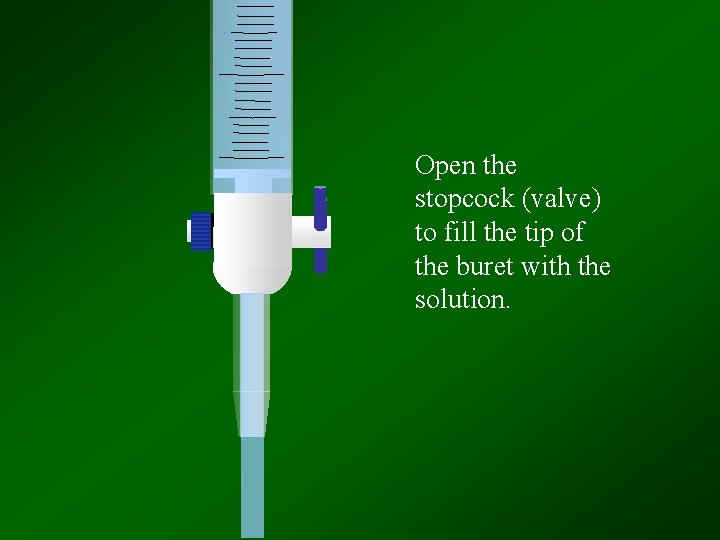
Open the stopcock (valve) to fill the tip of the buret with the solution.
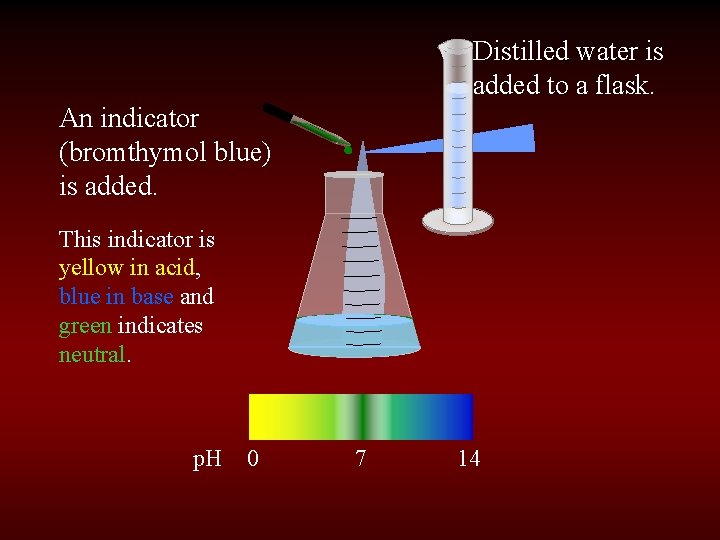
Distilled water is added to a flask. An indicator (bromthymol blue) is added. This indicator is yellow in acid, blue in base and green indicates neutral. p. H 0 7 14
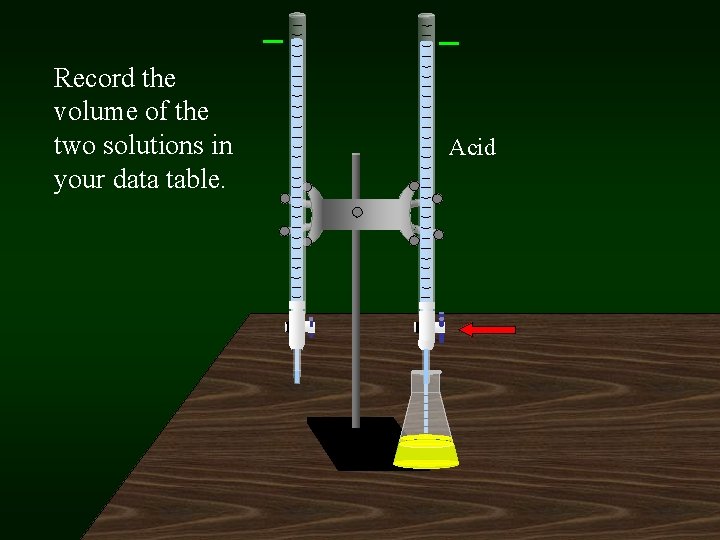
Record the volume of the two solutions in your data table. Acid
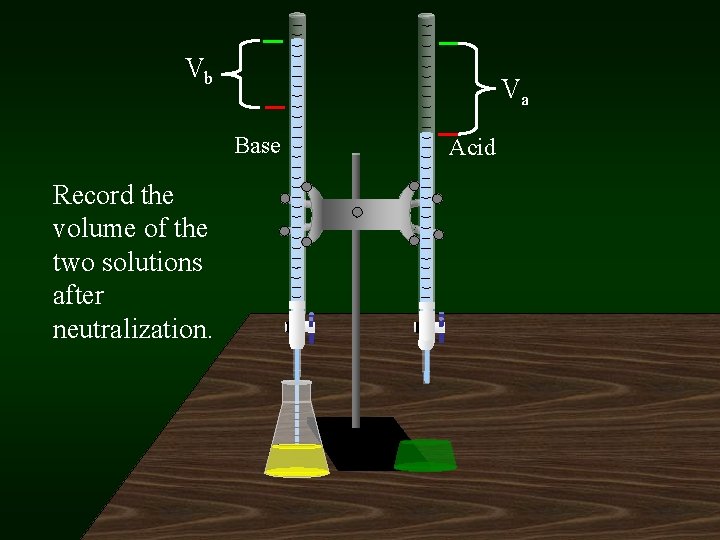
Vb Va Base Record the volume of the two solutions after neutralization. Acid
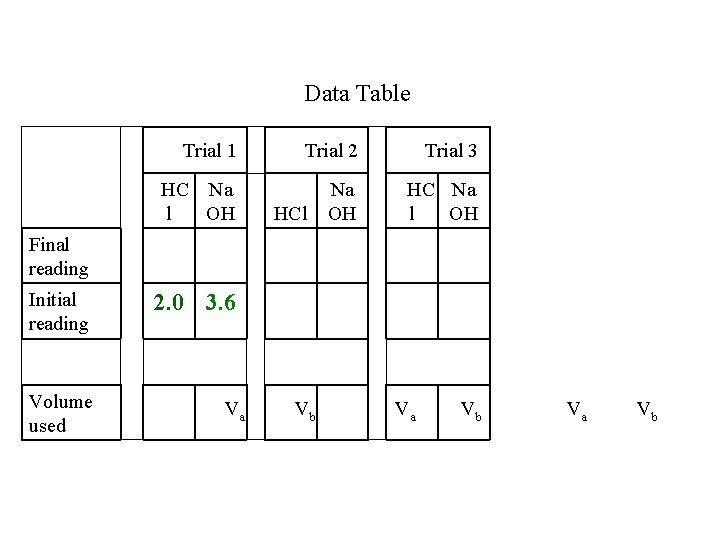
Data Table Trial 1 Trial 2 Trial 3 HC Na l OH Na OH HC Na l OH HCl Final reading Initial reading Volume used 2. 0 3. 6 Va Vb
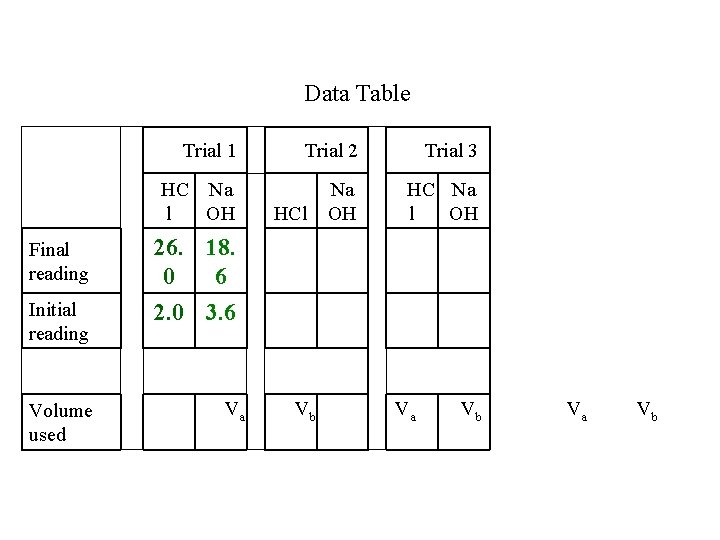
Data Table Trial 1 Trial 2 Trial 3 HC Na l OH Na OH HC Na l OH Final reading 26. 18. 0 6 Initial reading 2. 0 3. 6 Volume used Va HCl Vb Va Vb
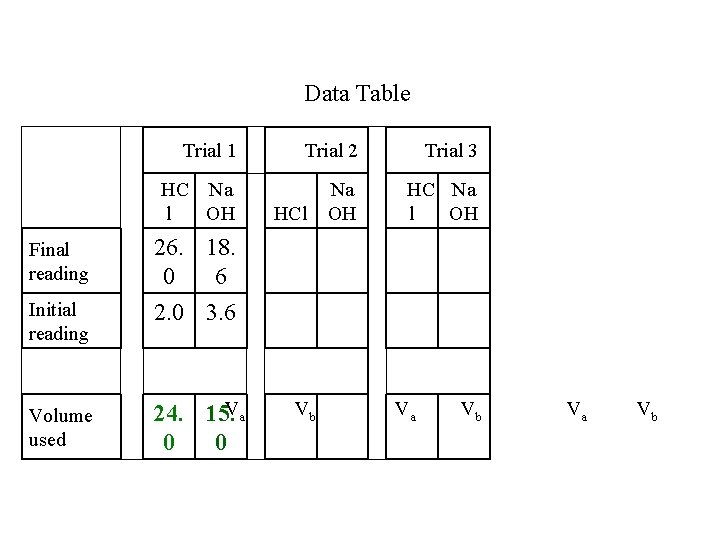
Data Table Trial 1 Trial 2 Trial 3 HC Na l OH Na OH HC Na l OH Final reading 26. 18. 0 6 Initial reading 2. 0 3. 6 Volume used 24. 15. Va 0 0 HCl Vb Va Vb
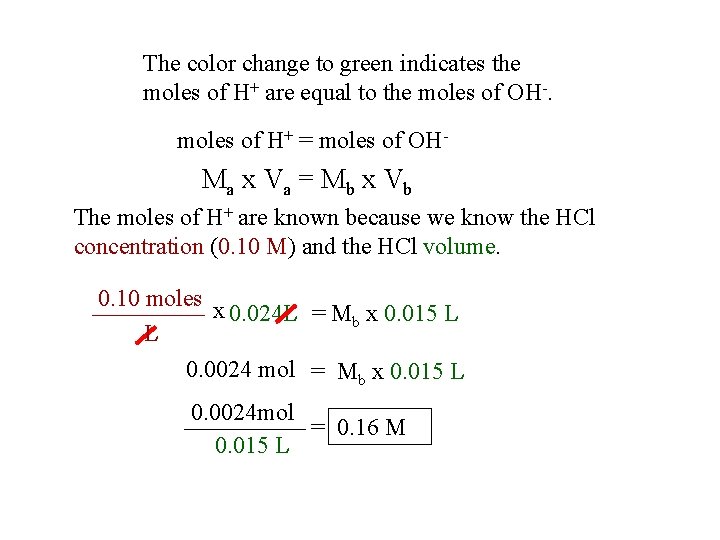
The color change to green indicates the moles of H+ are equal to the moles of OH-. moles of H+ = moles of OH- Ma x Va = M b x Vb The moles of H+ are known because we know the HCl concentration (0. 10 M) and the HCl volume. 0. 10 moles x 0. 024 L = Mb x 0. 015 L L 0. 0024 mol = Mb x 0. 015 L 0. 0024 mol = 0. 16 M 0. 015 L
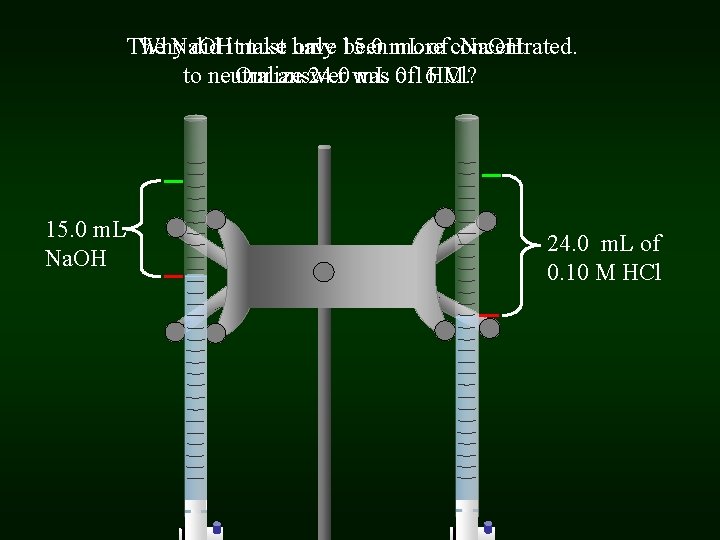
The Why Na. OH did itmust take have only 15. 0 been m. L more of concentrated. Na. OH to neutralize Our answer 24. 0 was m. L 0. 16 of HCl? M. 15. 0 m. L Na. OH 24. 0 m. L of 0. 10 M HCl
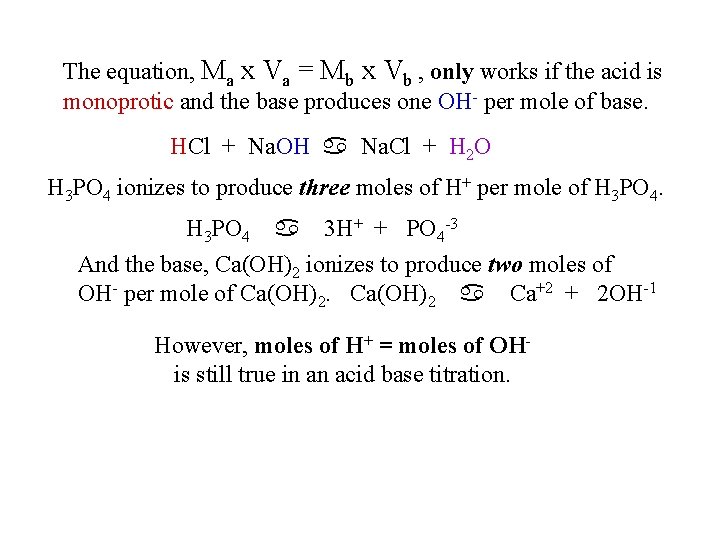
The equation, Ma x Va = Mb x Vb , only works if the acid is monoprotic and the base produces one OH- per mole of base. HCl + Na. OH a Na. Cl + H 2 O H 3 PO 4 ionizes to produce three moles of H+ per mole of H 3 PO 4 a 3 H+ + PO 4 -3 And the base, Ca(OH)2 ionizes to produce two moles of OH- per mole of Ca(OH)2 a Ca+2 + 2 OH-1 However, moles of H+ = moles of OHis still true in an acid base titration.
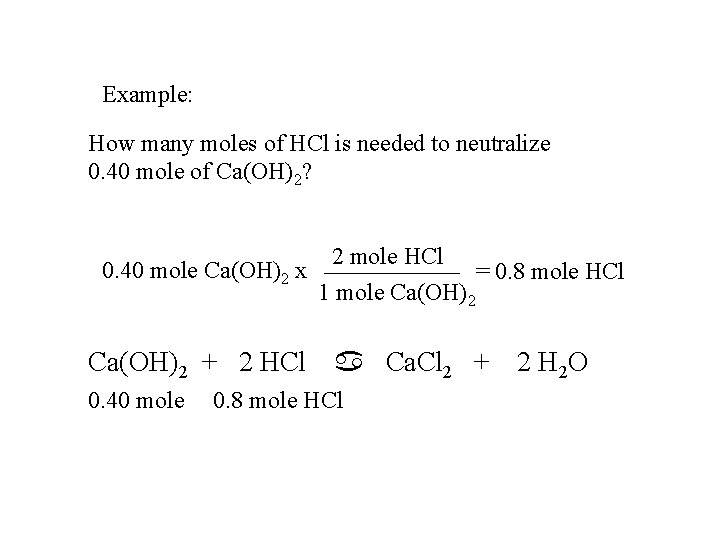
Example: How many moles of HCl is needed to neutralize 0. 40 mole of Ca(OH)2? 2 mole HCl 0. 40 mole Ca(OH)2 x = 0. 8 mole HCl 1 mole Ca(OH)2 + 2 HCl 0. 40 mole a Ca. Cl 2 + 2 H 2 O 0. 8 mole HCl
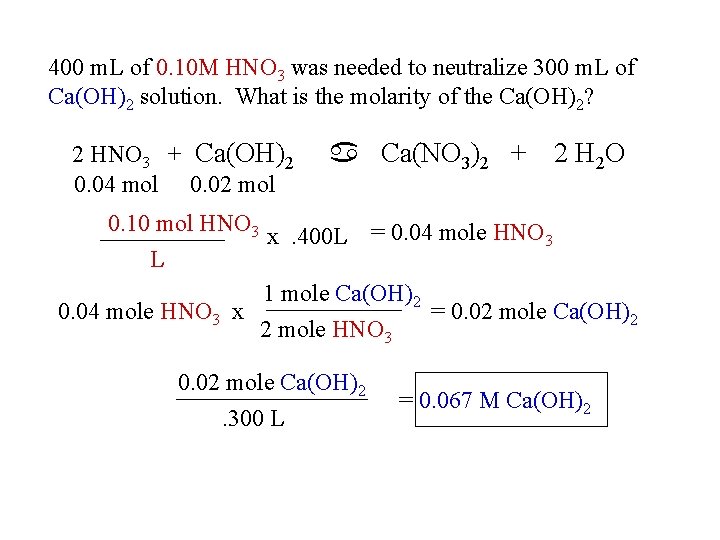
400 m. L of 0. 10 M HNO 3 was needed to neutralize 300 m. L of Ca(OH)2 solution. What is the molarity of the Ca(OH)2? 2 HNO 3 + Ca(OH)2 0. 04 mol 0. 02 mol 0. 10 mol HNO 3 L a Ca(NO 3)2 + 2 H 2 O x. 400 L = 0. 04 mole HNO 3 1 mole Ca(OH)2 0. 04 mole HNO 3 x = 0. 02 mole Ca(OH)2 2 mole HNO 3 0. 02 mole Ca(OH)2. 300 L = 0. 067 M Ca(OH)2
- Slides: 19