Ka Kb Comparing the p H of two

Ka, Kb
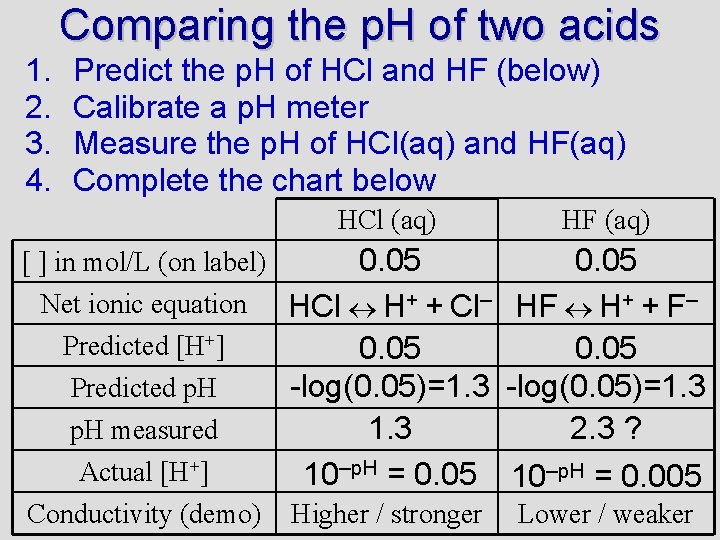
Comparing the p. H of two acids 1. 2. 3. 4. Predict the p. H of HCl and HF (below) Calibrate a p. H meter Measure the p. H of HCl(aq) and HF(aq) Complete the chart below HCl (aq) 0. 05 [ ] in mol/L (on label) Net ionic equation HCl H+ + Cl– Predicted [H+] 0. 05 -log(0. 05)=1. 3 Predicted p. H 1. 3 p. H measured Actual [H+] 10–p. H = 0. 05 Conductivity (demo) Higher / stronger HF (aq) 0. 05 HF H+ + F– 0. 05 -log(0. 05)=1. 3 2. 3 ? 10–p. H = 0. 005 Lower / weaker

Read 15. 3. (pg. 607+) Questions 1. Based on your results, which acid ionizes (forms ions) to a greater degree? 2. Which two measurements taken in the lab support your answer to 1? 3. What is another name for Ka? 4. Solve PE 5, 6 5. Write the Ka equation for HCl (aq) and HF (aq) from today’s lab 6. Solve for PE 8, 9 (use this equilibrium for butyric acid: HBu H+ + Bu–) 7. For HF(aq) set up a RICE chart, then solve for Ka. How does your value for Ka compare to the accepted value (pg. 608)? 8. Try PE 10 (follow example 15. 7 on pg. 610)

Answers 1. HCl ionizes more than HF 2. HCl has a lower p. H (indicating more H+), & a higher conductivity (indicating more ions) 3. Ka: acid ionization constant 4. HNO 2 H+ + NO 2–, Ka=[H+][NO 2–]/[HNO 2] HPO 42– H+ + PO 43–, Ka=[H+][PO 43–]/[HPO 42–] 5. HCl H+ + Cl–, Ka=[H+][Cl–]/[HCl] HF H+ + F–, Ka=[H+][F–]/[HF]
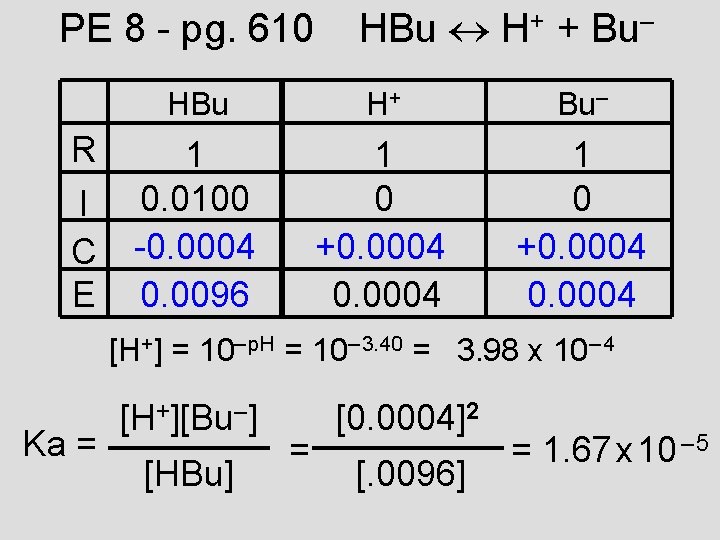
PE 8 - pg. 610 R I C E HBu H+ + Bu– HBu H+ Bu– 1 0. 0100 -0. 0004 0. 0096 1 0 +0. 0004 [H+] = 10– p. H = 10– 3. 40 = 3. 98 x 10– 4 Ka = [H+][Bu–] [HBu] = [0. 0004]2 [. 0096] = 1. 67 x 10 – 5
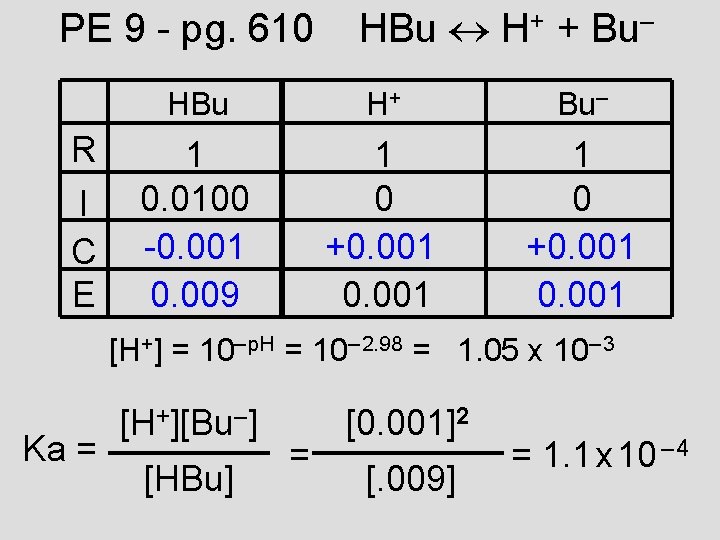
PE 9 - pg. 610 R I C E HBu H+ + Bu– HBu H+ Bu– 1 0. 0100 -0. 001 0. 009 1 0 +0. 001 [H+] = 10– p. H = 10– 2. 98 = 1. 05 x 10– 3 Ka = [H+][Bu–] [HBu] = [0. 001]2 [. 009] = 1. 1 x 10 – 4

Question 7: HF H+ + F– R I C E HF H+ F– 1 0. 05 -0. 005 0. 045 1 0 +0. 005 [H+] = 10– p. H = 10– 2. 3 = 0. 005 Ka = [H+][F–] [HF] = [0. 005]2 [. 045] = 5. 6 x 10 – 4 Accepted value of Ka for HF is 6. 4 x 10 – 4
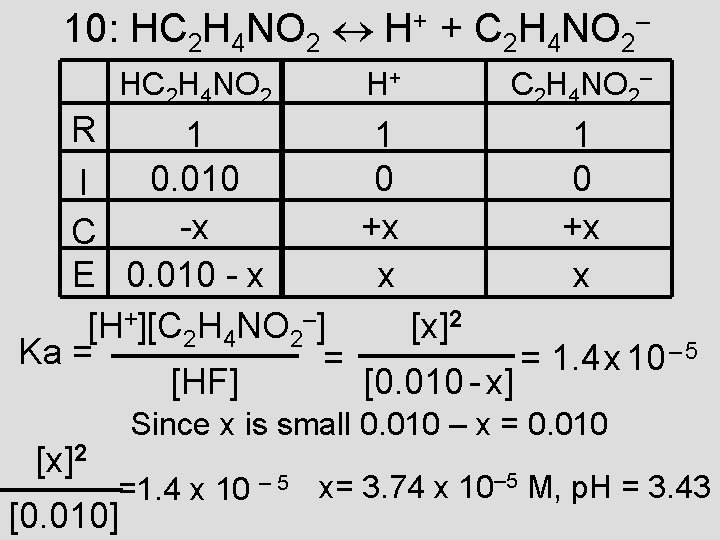
10: HC 2 H 4 NO 2 H+ + C 2 H 4 NO 2– HC 2 H 4 NO 2 R 1 0. 010 I -x C E 0. 010 - x [H+][C 2 H 4 NO 2–] Ka = = [HF] [x]2 H+ C 2 H 4 NO 2– 1 0 +x x [x]2 [0. 010 - x] = 1. 4 x 10 – 5 Since x is small 0. 010 – x = 0. 010 =1. 4 x 10 – 5 x= 3. 74 x 10– 5 M, p. H = 3. 43 [0. 010]

Ka summary • • • Ka follows the pattern of other “K” equations I. e. for HA(aq) + H 2 O(l) H 3 O+(aq) + A–(aq) Ka = [H 3 O+][A–] / [HA] Notice that H 2 O is ignored because it is liquid HA cannot be ignored because it is aqueous This is different than with Ksp. In Ksp, solids could only be in solution as ions • Acids can be in solution whether ionized or not • The solubility of acids makes sense if you think back to the partial charges in HCl for ex.

Ka summary • Generally Ka tells you about acid strength • Strong acids have high Ka values • A “strong” acid is an acid that completely ionizes. E. g. HCl + H 2 O H 3 O+ + Cl– • A “weak” acid is an acid that doesn’t ionize completely. E. g. HF + H 2 O H 3 O+ + F– • Note: don’t get confused between strength and concentration. 1 M HCN has a smaller [H+], thus a higher p. H, than 0. 001 M HCl • In general: Ka < 10 – 3 Weak acid 10 – 3 < Ka < 1 Moderate acid Ka > 1 Strong acid
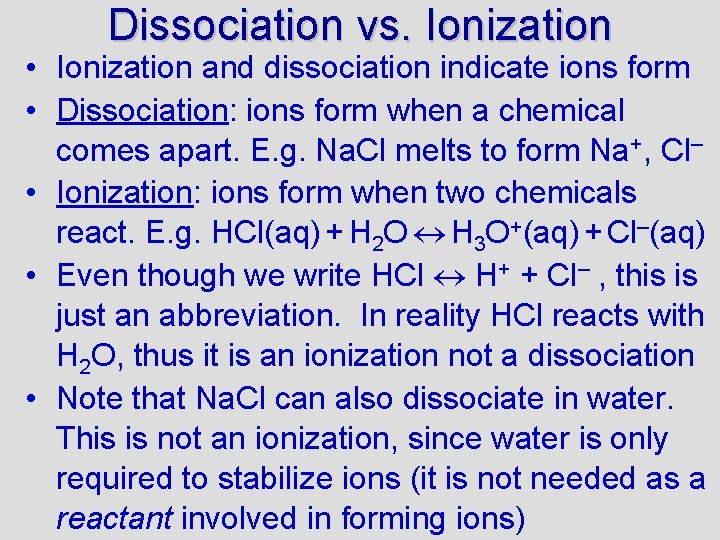
Dissociation vs. Ionization • Ionization and dissociation indicate ions form • Dissociation: ions form when a chemical comes apart. E. g. Na. Cl melts to form Na+, Cl– • Ionization: ions form when two chemicals react. E. g. HCl(aq) + H 2 O H 3 O+(aq) + Cl–(aq) • Even though we write HCl H+ + Cl– , this is just an abbreviation. In reality HCl reacts with H 2 O, thus it is an ionization not a dissociation • Note that Na. Cl can also dissociate in water. This is not an ionization, since water is only required to stabilize ions (it is not needed as a reactant involved in forming ions)

Kb – the last K (I promise) • Kb is similar to Ka except b stands for base • The general reaction involving a base can be written as B(aq) + H 2 O BH+(aq) + OH–(aq) • Thus Kb = [BH+] [OH–] / [B] • Recall: shorthand for Ka is HA H+ + A– • Kb has no shorthand form • Read pg. 614 - 617 • Try PE 12 (a-c), 13, 14 (for 13, you do not need to know the chemical formula of morphine. Symbolize it with M)
![PE 12 a) CN–(aq) + H 2 O HCN(aq) + OH–(aq) Kb = [HCN][OH–] PE 12 a) CN–(aq) + H 2 O HCN(aq) + OH–(aq) Kb = [HCN][OH–]](http://slidetodoc.com/presentation_image_h2/786f865166a5ad68d14ffd92306239ec/image-13.jpg)
PE 12 a) CN–(aq) + H 2 O HCN(aq) + OH–(aq) Kb = [HCN][OH–] / [CN–] b) C 2 H 3 O 2–(aq) + H 2 O HC 2 H 3 O 2(aq) + OH–(aq) Kb = [HC 2 H 3 O 2][OH–] / [C 2 H 3 O 2–] c) C 6 H 5 NH 2(aq) + H 2 O C 6 H 5 NH 3+(aq) + OH–(aq) Kb = [C 6 H 5 NH 3+][OH–] / [C 6 H 5 NH 2]
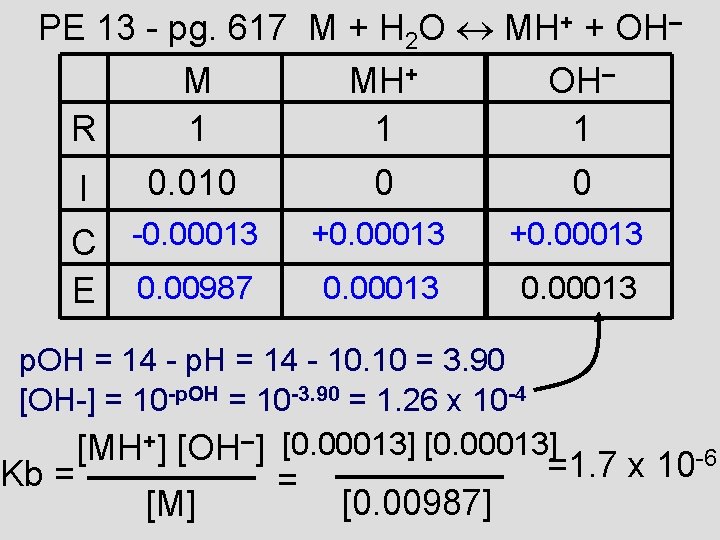
PE 13 - pg. 617 M + H 2 O MH+ + OH– M MH+ OH– R 1 1 1 0. 010 I C -0. 00013 E 0. 00987 0 0 +0. 00013 p. OH = 14 - p. H = 14 - 10. 10 = 3. 90 [OH-] = 10 -p. OH = 10 -3. 90 = 1. 26 x 10 -4 [MH+] [OH–] [0. 00013] Kb = [M] = [0. 00987] =1. 7 x 10 -6
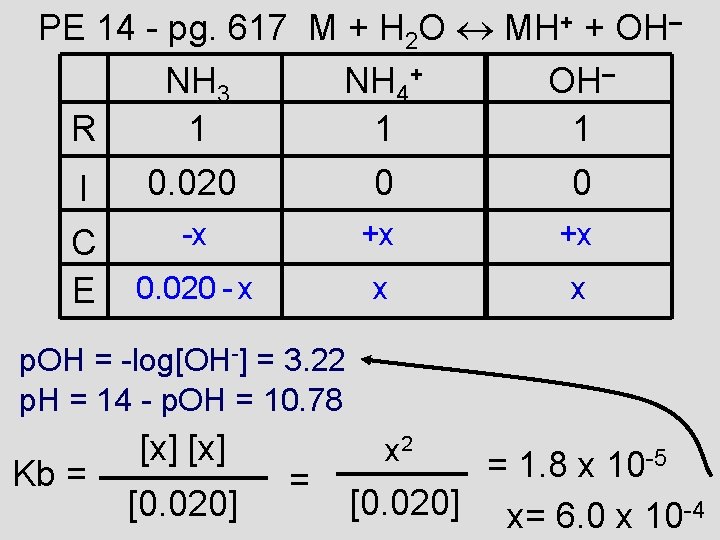
PE 14 - pg. 617 M + H 2 O MH+ + OH– NH 3 NH 4+ OH– R 1 1 1 I C E 0. 020 0 0 -x +x +x 0. 020 - x x x p. OH = -log[OH-] = 3. 22 p. H = 14 - p. OH = 10. 78 Kb = [x] [0. 020] = x 2 = 1. 8 x 10 -5 [0. 020] x= 6. 0 x 10 -4

Strength of conjugates Consider HCl(l) + H 2 O Cl–(aq) + H 3 O+(aq) The Ka for HCl is [Cl–(aq)] [H 3 O+(aq)] / [HCl(aq)] Also, Cl–(aq) + H 2 O(aq) HCl(l) + OH– The Kb for Cl– is [HCl(aq)] / [Cl–(aq)] [H 3 O+(aq)]
![Relative values of Ka Recall for HX H+ + X–, Ka = [H+][X–] / Relative values of Ka Recall for HX H+ + X–, Ka = [H+][X–] /](http://slidetodoc.com/presentation_image_h2/786f865166a5ad68d14ffd92306239ec/image-17.jpg)
Relative values of Ka Recall for HX H+ + X–, Ka = [H+][X–] / [HX] Q - what does a large Ka indicate? A - equilibrium is far to the right (all dissociates) Thus a large Ka = strong acid Look at Table 15. 4 on page 608 The text uses this definition: Ka < 10– 3 is a weak acid 10– 3 < Ka < 1 is a moderate acid 1 < Ka is a strong acid These definitions are somewhat arbitrary, we will not focus on this. Just remember a high Ka means the acid is strong. For more lessons, visit www. chalkbored. com
- Slides: 17