Ka Kb Acid and Base Dissociation Constants How

Ka, Kb Acid and Base Dissociation Constants
![How do we calculate [H+] for a weak acid? �We know that strong acids How do we calculate [H+] for a weak acid? �We know that strong acids](http://slidetodoc.com/presentation_image_h2/6c180dc591ae5f6ba19482577e1f3442/image-2.jpg)
How do we calculate [H+] for a weak acid? �We know that strong acids dissociate 100% and that, therefore, the [H+] equals that [acid] that we start with �What about weak acids? �Don’t ionize 100%, so the [H+] is NOT the same as our starting concentration of our acid!

Recall Kw �Autoionization of water: H 2 O ↔ H+ + OHOr H 2 O + H 2 O ↔ H 3 O+ + OH�Keq = �Kw = [H 3 O+] [OH-] = 1. 0 x 10 -14
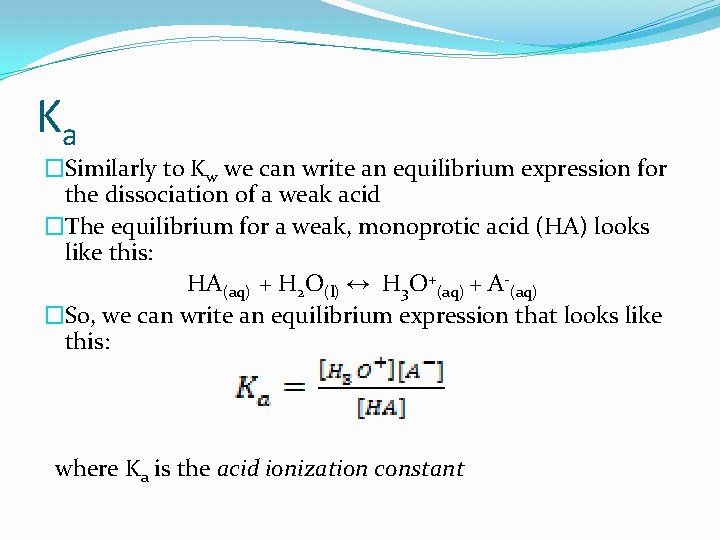
Ka �Similarly to Kw we can write an equilibrium expression for the dissociation of a weak acid �The equilibrium for a weak, monoprotic acid (HA) looks like this: HA(aq) + H 2 O(l) ↔ H 3 O+(aq) + A-(aq) �So, we can write an equilibrium expression that looks like this: where Ka is the acid ionization constant
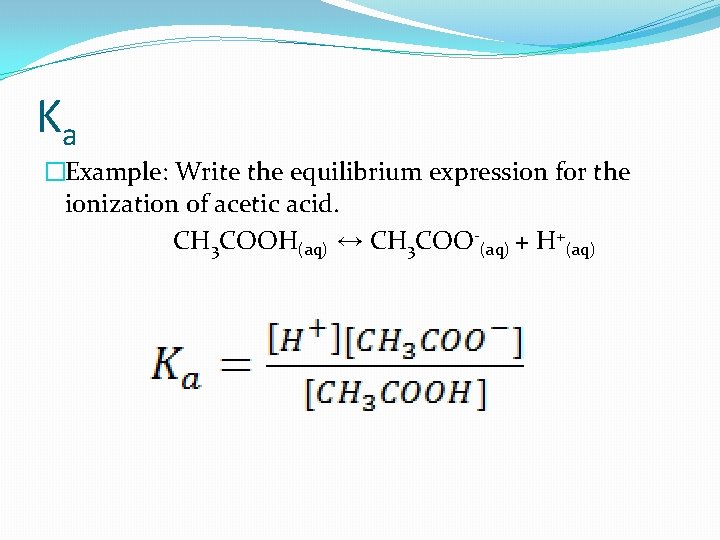
Ka �Example: Write the equilibrium expression for the ionization of acetic acid. CH 3 COOH(aq) ↔ CH 3 COO-(aq) + H+(aq)

Ka and Acid Strength �Ka values are typically between 1 – 1 x 10 -16 �The higher the value of Ka, the more the acid dissociates in water and, hence, the stronger the acid

What about weak bases? �Weak bases also form an equilibrium in water: B(aq) + H 2 O(l) ↔ HB+(aq) + OH-(aq) �This can be represented by the base dissociation constant: �Like Ka, a higher Kb means that more B has dissociated and, therefore, the stronger the base

Note: Coefficients and Equilibrium Expressions �If you have coefficients in your reaction equation, they become subscripts in the equilibrium expression: 2 AB → A 2 + B 2

Try it �Write the equilibrium expression for the dissociation of NH 3 in water. NH 3(aq) + H 2 O(l) ↔ NH 4(aq)+ + OH-(aq) Try the Self Test 10. 2
![So how does Ka help us find the [H+]? �The Ka’s for almost every So how does Ka help us find the [H+]? �The Ka’s for almost every](http://slidetodoc.com/presentation_image_h2/6c180dc591ae5f6ba19482577e1f3442/image-10.jpg)
So how does Ka help us find the [H+]? �The Ka’s for almost every weak acid you could think of have been measured (at 25 o. C) and recorded �If we know the value of Ka and the starting concentration of our weak acid, we can solve for [H+]

Try It: �What is the concentration of H+ in 0. 50 M HF at 25 o. C? �From the acid table, Ka = 7. 1 x 10 -4, so: HF(aq) ↔ H+(aq) + F-(aq) �Now what? Now, we use ICE tables!

ICE table HF(aq) ↔ H+(aq) + F-(aq) � Initial (M) Change (M) Equilibrium (M) 0. 50 0 0 -x +x +x 0. 50 – x x x
![Solve for x �In this case x is our [H+] Solve for x �In this case x is our [H+]](http://slidetodoc.com/presentation_image_h2/6c180dc591ae5f6ba19482577e1f3442/image-13.jpg)
Solve for x �In this case x is our [H+]

Short Cut: �If < 500, the change in the initial concentration (x) is negligible and can be ignored. HF(aq) ↔ Initial (M) Change (M) Equilibrium (M) H+(aq) + F-(aq) 0. 50 0 0 -x +x +x 0. 50 – x x x ]

Percent Dissociation (aka. Percent Ionization) �The fraction of molecules that dissociate compared to the initial concentration, expressed as a percent: �Percent dissociation = �Ex: If a 0. 10 M solution of benzoic acid was found to dissociate to give a [H+] = 1. 1 x 10 -3 M, the percent dissociation would be:
- Slides: 15