Jyvskyl Summer School on Charge Density August 2007


- Slides: 48

Jyväskylä Summer School on Charge Density August 2007 Chemical Applications of Charge Density Louis J Farrugia

Jyväskylä Summer School on Charge Density August 2007 Chemical applications of charge density 1. VSEPR rules and the Laplacian 2. Hydrogen bonding 3. Metal-metal bonding

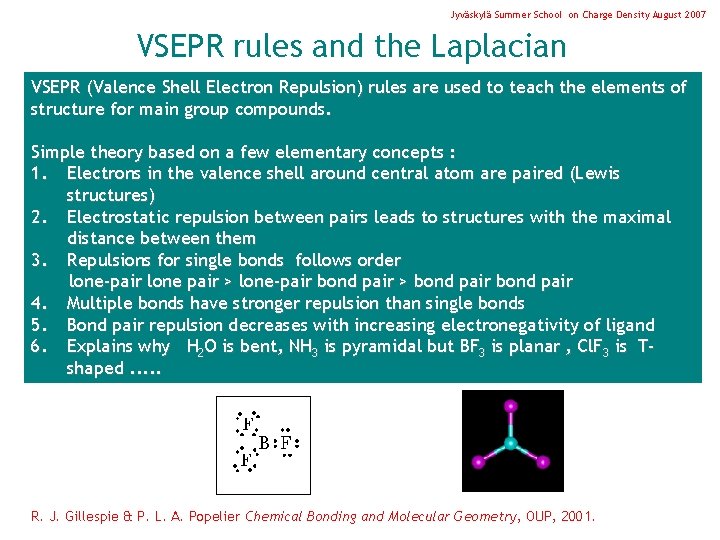
Jyväskylä Summer School on Charge Density August 2007 VSEPR rules and the Laplacian VSEPR (Valence Shell Electron Repulsion) rules are used to teach the elements of structure for main group compounds. Simple theory based on a few elementary concepts : 1. Electrons in the valence shell around central atom are paired (Lewis structures) 2. Electrostatic repulsion between pairs leads to structures with the maximal distance between them 3. Repulsions for single bonds follows order lone-pair lone pair > lone-pair bond pair > bond pair 4. Multiple bonds have stronger repulsion than single bonds 5. Bond pair repulsion decreases with increasing electronegativity of ligand 6. Explains why H 2 O is bent, NH 3 is pyramidal but BF 3 is planar , Cl. F 3 is Tshaped. . . R. J. Gillespie & P. L. A. Popelier Chemical Bonding and Molecular Geometry, OUP, 2001.

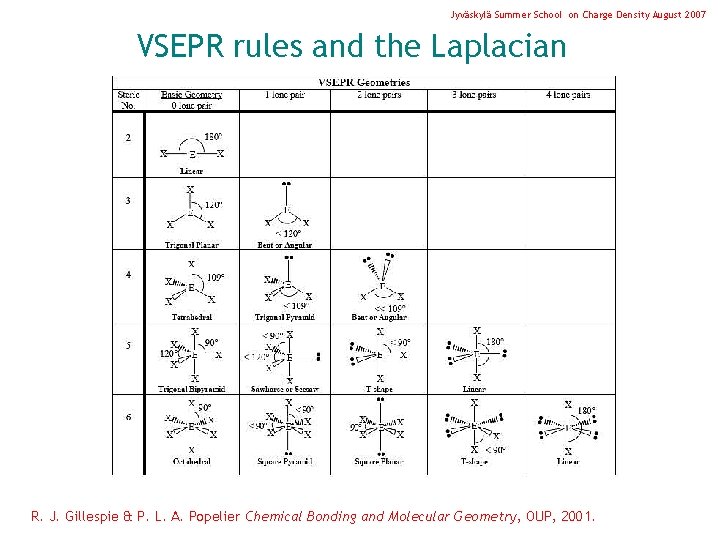
Jyväskylä Summer School on Charge Density August 2007 VSEPR rules and the Laplacian R. J. Gillespie & P. L. A. Popelier Chemical Bonding and Molecular Geometry, OUP, 2001.

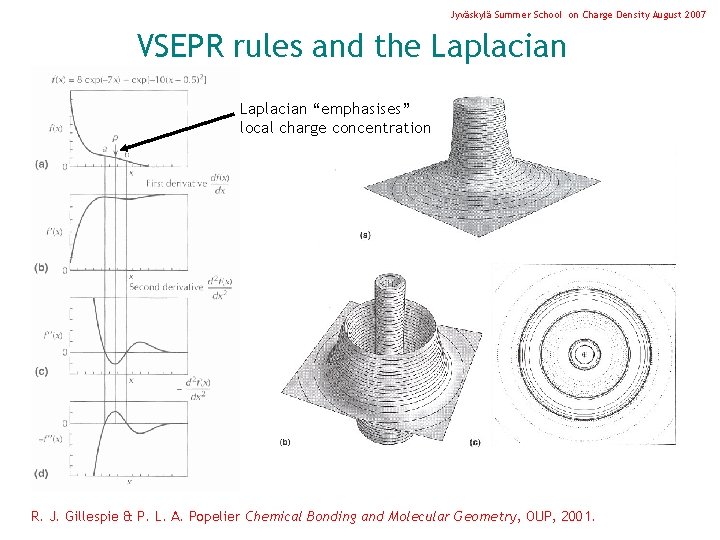
Jyväskylä Summer School on Charge Density August 2007 VSEPR rules and the Laplacian “emphasises” local charge concentration R. J. Gillespie & P. L. A. Popelier Chemical Bonding and Molecular Geometry, OUP, 2001.

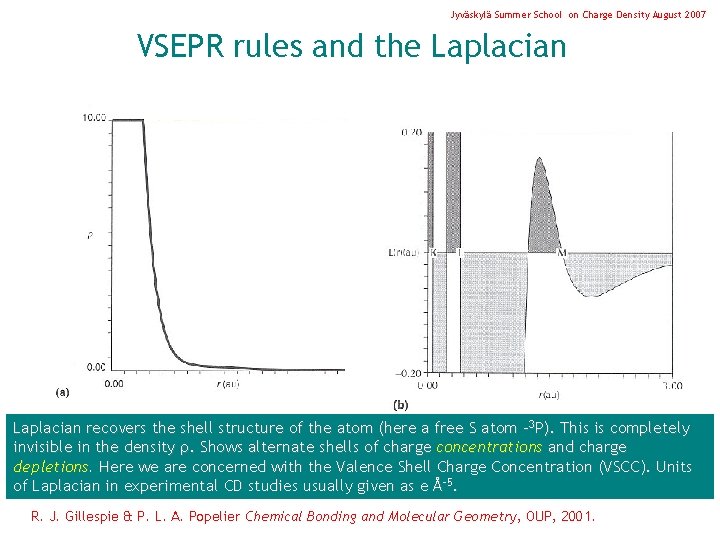
Jyväskylä Summer School on Charge Density August 2007 VSEPR rules and the Laplacian recovers the shell structure of the atom (here a free S atom - 3 P). This is completely invisible in the density . Shows alternate shells of charge concentrations and charge depletions. Here we are concerned with the Valence Shell Charge Concentration (VSCC). Units of Laplacian in experimental CD studies usually given as e Å-5. R. J. Gillespie & P. L. A. Popelier Chemical Bonding and Molecular Geometry, OUP, 2001.

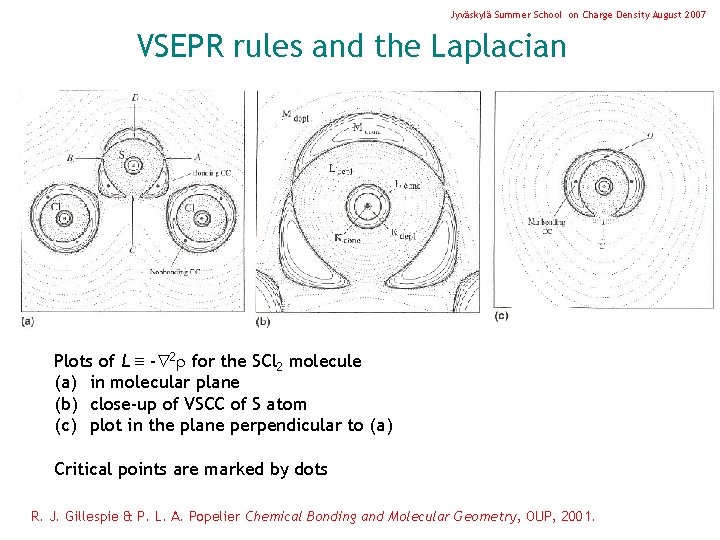
Jyväskylä Summer School on Charge Density August 2007 VSEPR rules and the Laplacian Plots of L - 2 for the SCl 2 molecule (a) in molecular plane (b) close-up of VSCC of S atom (c) plot in the plane perpendicular to (a) Critical points are marked by dots R. J. Gillespie & P. L. A. Popelier Chemical Bonding and Molecular Geometry, OUP, 2001.

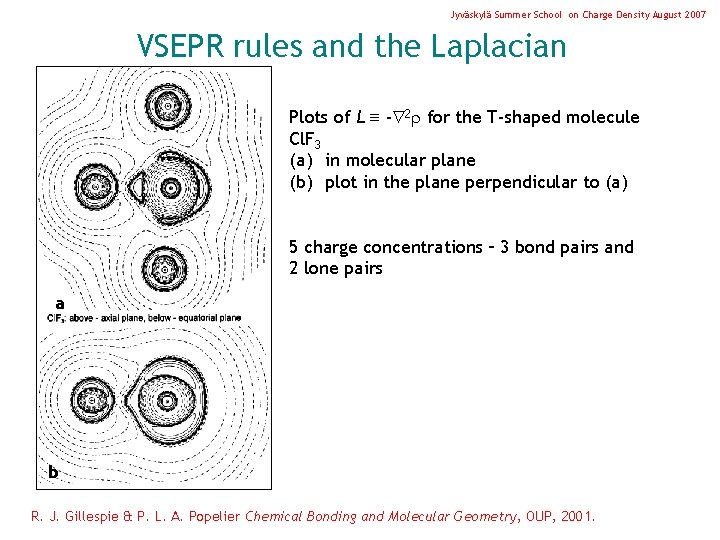
Jyväskylä Summer School on Charge Density August 2007 VSEPR rules and the Laplacian Plots of L - 2 for the T-shaped molecule Cl. F 3 (a) in molecular plane (b) plot in the plane perpendicular to (a) 5 charge concentrations – 3 bond pairs and 2 lone pairs a b R. J. Gillespie & P. L. A. Popelier Chemical Bonding and Molecular Geometry, OUP, 2001.

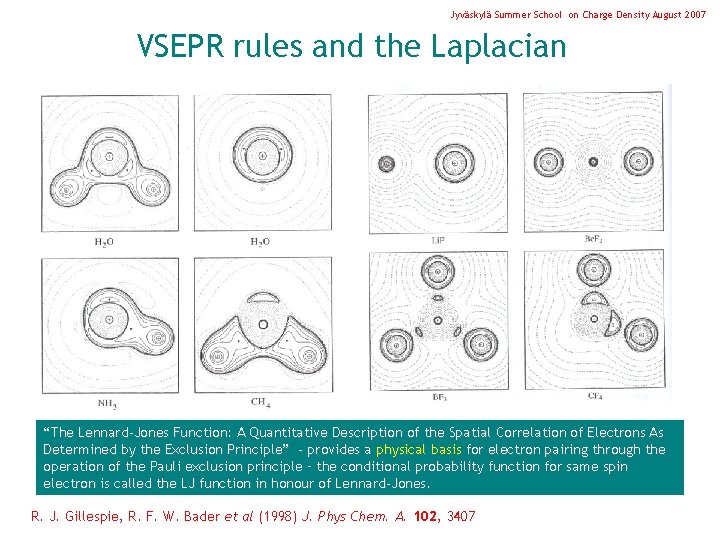
Jyväskylä Summer School on Charge Density August 2007 VSEPR rules and the Laplacian “The Lennard-Jones Function: A Quantitative Description of the Spatial Correlation of Electrons As Determined by the Exclusion Principle” - provides a physical basis for electron pairing through the operation of the Pauli exclusion principle – the conditional probability function for same spin electron is called the LJ function in honour of Lennard-Jones. R. J. Gillespie, R. F. W. Bader et al (1998) J. Phys Chem. A. 102, 3407

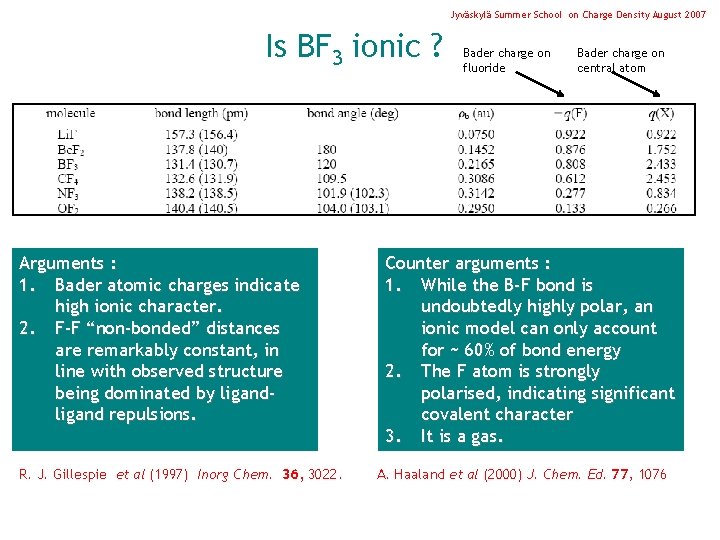
Jyväskylä Summer School on Charge Density August 2007 Is BF 3 ionic ? Arguments : 1. Bader atomic charges indicate high ionic character. 2. F-F “non-bonded” distances are remarkably constant, in line with observed structure being dominated by ligand repulsions. R. J. Gillespie et al (1997) Inorg Chem. 36, 3022. Bader charge on fluoride Bader charge on central atom Counter arguments : 1. While the B-F bond is undoubtedly highly polar, an ionic model can only account for ~ 60% of bond energy 2. The F atom is strongly polarised, indicating significant covalent character 3. It is a gas. A. Haaland et al (2000) J. Chem. Ed. 77, 1076

Jyväskylä Summer School on Charge Density August 2007 Hydrogen bonds were first described (probably) by M. L. Huggins in 1919, but Linus Pauling (1931) was the first to coin the term hydrogen bond to describe the bonding in the [F-H. . . F]- anion. G. A. Jeffrey (1997) An Introduction to Hydrogen Bonding, OUP, New York.

Jyväskylä Summer School on Charge Density August 2007 Hydrogen bonds G. Gilli, P. Gilli (2000) “Towards a Unified H-bond Theory” J. Mol. Struct 552, 1

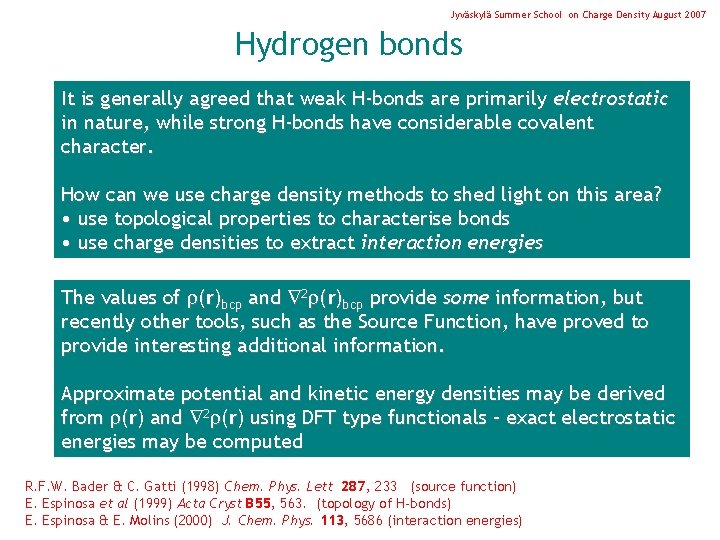
Jyväskylä Summer School on Charge Density August 2007 Hydrogen bonds It is generally agreed that weak H-bonds are primarily electrostatic in nature, while strong H-bonds have considerable covalent character. How can we use charge density methods to shed light on this area? • use topological properties to characterise bonds • use charge densities to extract interaction energies The values of (r)bcp and 2 (r)bcp provide some information, but recently other tools, such as the Source Function, have proved to provide interesting additional information. Approximate potential and kinetic energy densities may be derived from (r) and 2 (r) using DFT type functionals – exact electrostatic energies may be computed R. F. W. Bader & C. Gatti (1998) Chem. Phys. Lett 287, 233 (source function) E. Espinosa et al (1999) Acta Cryst B 55, 563. (topology of H-bonds) E. Espinosa & E. Molins (2000) J. Chem. Phys. 113, 5686 (interaction energies)

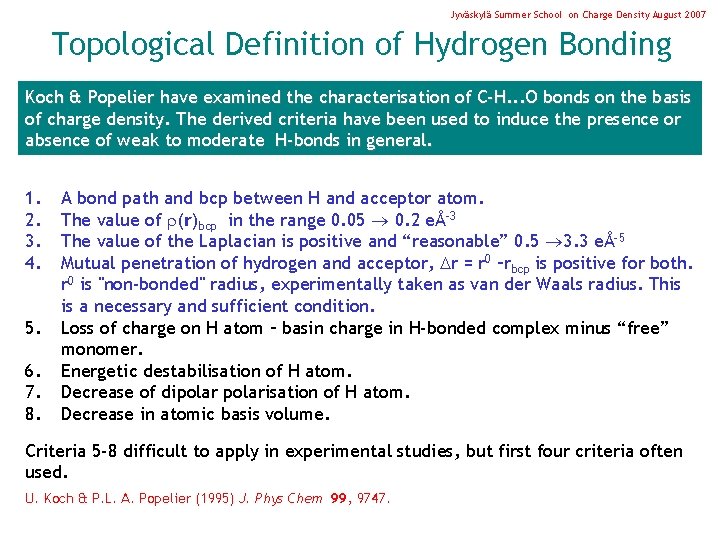
Jyväskylä Summer School on Charge Density August 2007 Topological Definition of Hydrogen Bonding Koch & Popelier have examined the characterisation of C-H. . . O bonds on the basis of charge density. The derived criteria have been used to induce the presence or absence of weak to moderate H-bonds in general. 1. 2. 3. 4. 5. 6. 7. 8. A bond path and bcp between H and acceptor atom. The value of (r)bcp in the range 0. 05 0. 2 eÅ-3 The value of the Laplacian is positive and “reasonable” 0. 5 3. 3 eÅ-5 Mutual penetration of hydrogen and acceptor, r = r 0 –rbcp is positive for both. r 0 is "non-bonded" radius, experimentally taken as van der Waals radius. This is a necessary and sufficient condition. Loss of charge on H atom – basin charge in H-bonded complex minus “free” monomer. Energetic destabilisation of H atom. Decrease of dipolarisation of H atom. Decrease in atomic basis volume. Criteria 5 -8 difficult to apply in experimental studies, but first four criteria often used. U. Koch & P. L. A. Popelier (1995) J. Phys Chem 99, 9747.

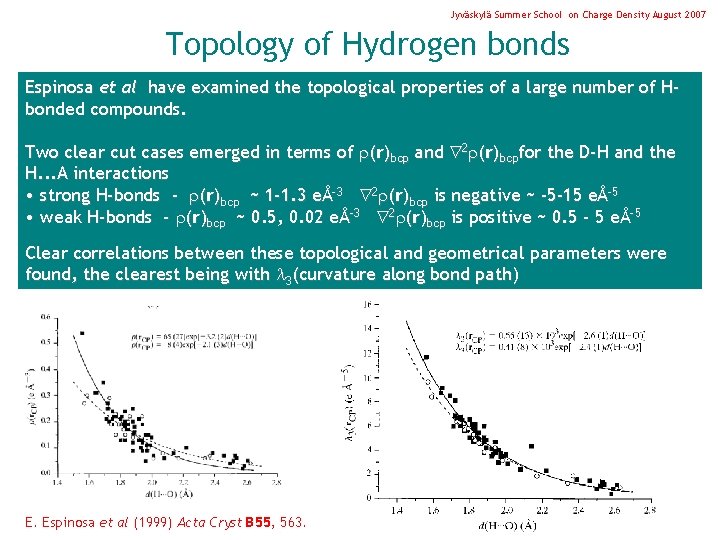
Jyväskylä Summer School on Charge Density August 2007 Topology of Hydrogen bonds Espinosa et al have examined the topological properties of a large number of Hbonded compounds. Two clear cut cases emerged in terms of (r)bcp and 2 (r)bcpfor the D-H and the H. . . A interactions • strong H-bonds - (r)bcp ~ 1 -1. 3 eÅ-3 2 (r)bcp is negative ~ -5 -15 eÅ-5 • weak H-bonds - (r)bcp ~ 0. 5, 0. 02 eÅ-3 2 (r)bcp is positive ~ 0. 5 - 5 eÅ-5 Clear correlations between these topological and geometrical parameters were found, the clearest being with 3(curvature along bond path) E. Espinosa et al (1999) Acta Cryst B 55, 563.

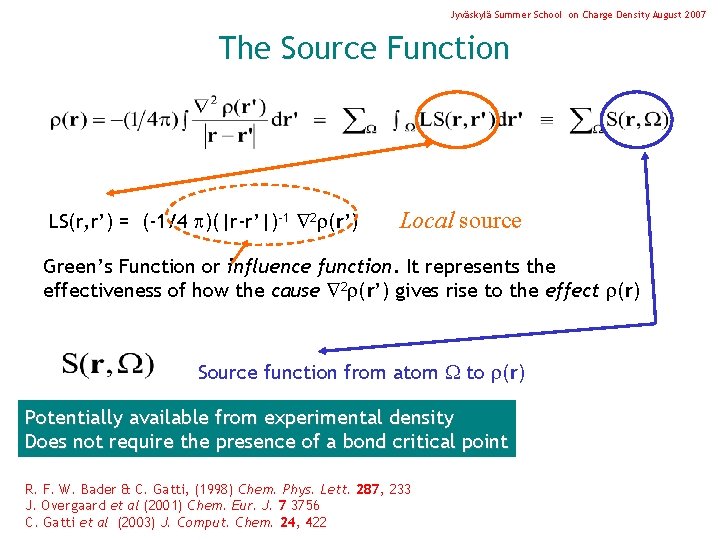
Jyväskylä Summer School on Charge Density August 2007 The Source Function LS(r, r’) = (-1/4 )(|r-r’|)-1 2 (r’) Local source Green’s Function or influence function. It represents the effectiveness of how the cause 2 (r’) gives rise to the effect (r) Source function from atom to (r) Potentially available from experimental density Does not require the presence of a bond critical point R. F. W. Bader & C. Gatti, (1998) Chem. Phys. Lett. 287, 233 J. Overgaard et al (2001) Chem. Eur. J. 7 3756 C. Gatti et al (2003) J. Comput. Chem. 24, 422

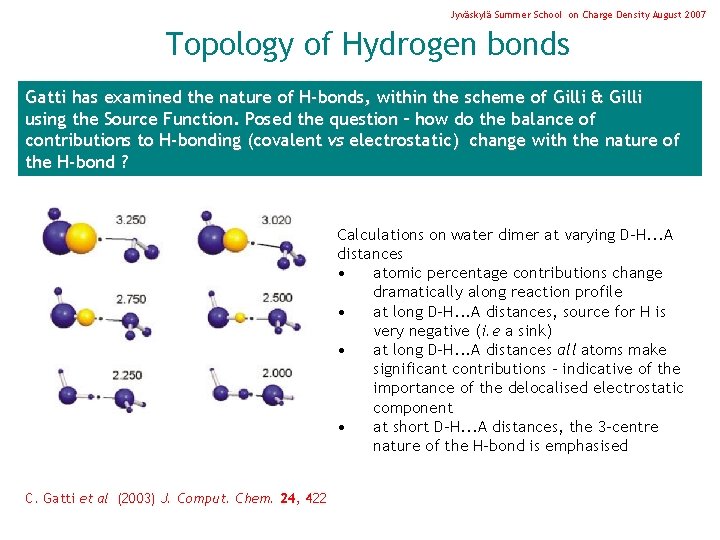
Jyväskylä Summer School on Charge Density August 2007 Topology of Hydrogen bonds Gatti has examined the nature of H-bonds, within the scheme of Gilli & Gilli using the Source Function. Posed the question – how do the balance of contributions to H-bonding (covalent vs electrostatic) change with the nature of the H-bond ? Calculations on water dimer at varying D-H. . . A distances • atomic percentage contributions change dramatically along reaction profile • at long D-H. . . A distances, source for H is very negative (i. e a sink) • at long D-H. . . A distances all atoms make significant contributions – indicative of the importance of the delocalised electrostatic component • at short D-H. . . A distances, the 3 -centre nature of the H-bond is emphasised C. Gatti et al (2003) J. Comput. Chem. 24, 422

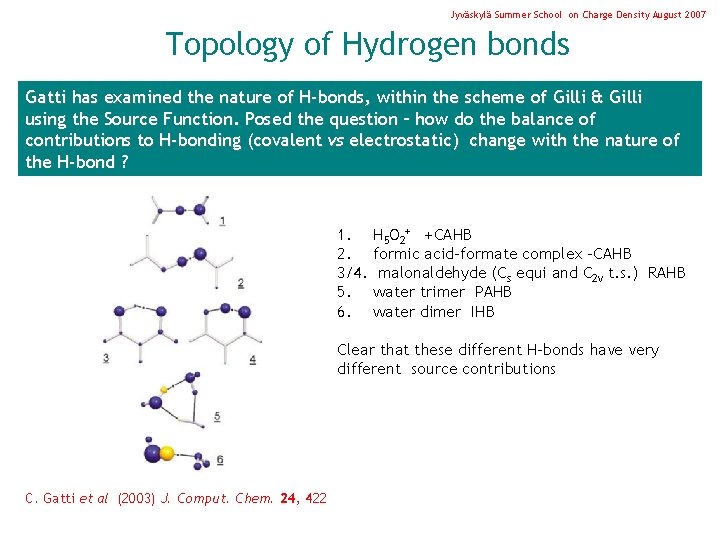
Jyväskylä Summer School on Charge Density August 2007 Topology of Hydrogen bonds Gatti has examined the nature of H-bonds, within the scheme of Gilli & Gilli using the Source Function. Posed the question – how do the balance of contributions to H-bonding (covalent vs electrostatic) change with the nature of the H-bond ? 1. 2. 3/4. 5. 6. H 5 O 2+ +CAHB formic acid-formate complex –CAHB malonaldehyde (Cs equi and C 2 v t. s. ) RAHB water trimer PAHB water dimer IHB Clear that these different H-bonds have very different source contributions C. Gatti et al (2003) J. Comput. Chem. 24, 422

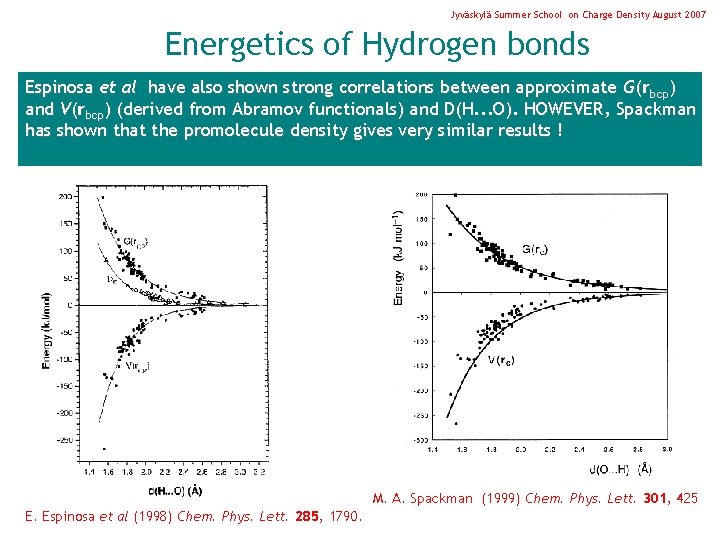
Jyväskylä Summer School on Charge Density August 2007 Energetics of Hydrogen bonds Espinosa et al have also shown strong correlations between approximate G(rbcp) and V(rbcp) (derived from Abramov functionals) and D(H. . . O). HOWEVER, Spackman has shown that the promolecule density gives very similar results ! M. A. Spackman (1999) Chem. Phys. Lett. 301, 425 E. Espinosa et al (1998) Chem. Phys. Lett. 285, 1790.

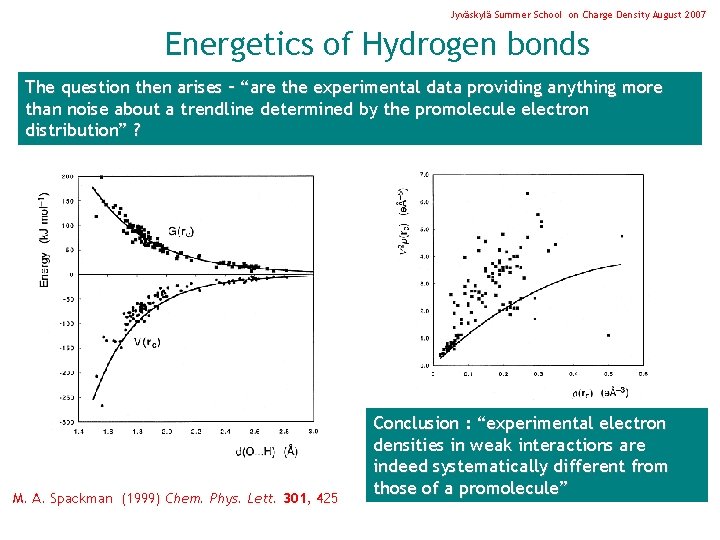
Jyväskylä Summer School on Charge Density August 2007 Energetics of Hydrogen bonds The question then arises – “are the experimental data providing anything more than noise about a trendline determined by the promolecule electron distribution” ? M. A. Spackman (1999) Chem. Phys. Lett. 301, 425 Conclusion : “experimental electron densities in weak interactions are indeed systematically different from those of a promolecule”

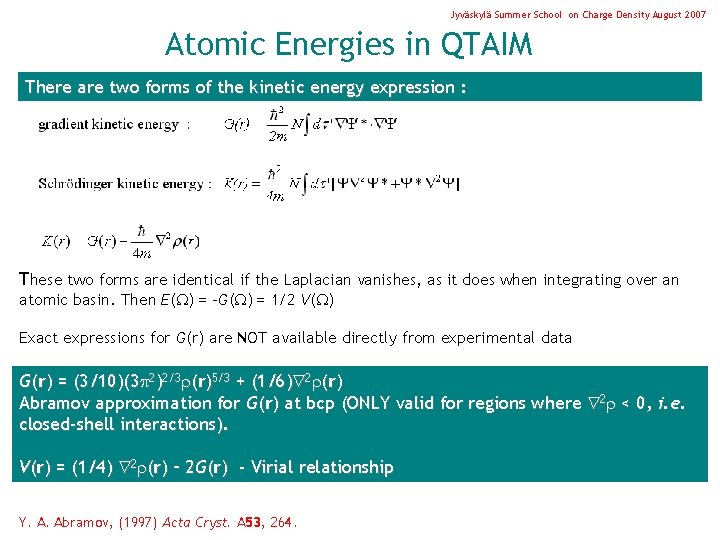
Jyväskylä Summer School on Charge Density August 2007 Atomic Energies in QTAIM There are two forms of the kinetic energy expression : These two forms are identical if the Laplacian vanishes, as it does when integrating over an atomic basin. Then E( ) = -G( ) = 1/2 V( ) Exact expressions for G(r) are NOT available directly from experimental data G(r) = (3/10)(3 2)2/3 (r)5/3 + (1/6) 2 (r) Abramov approximation for G(r) at bcp (ONLY valid for regions where 2 < 0, i. e. closed-shell interactions). V(r) = (1/4) 2 (r) – 2 G(r) - Virial relationship Y. A. Abramov, (1997) Acta Cryst. A 53, 264.

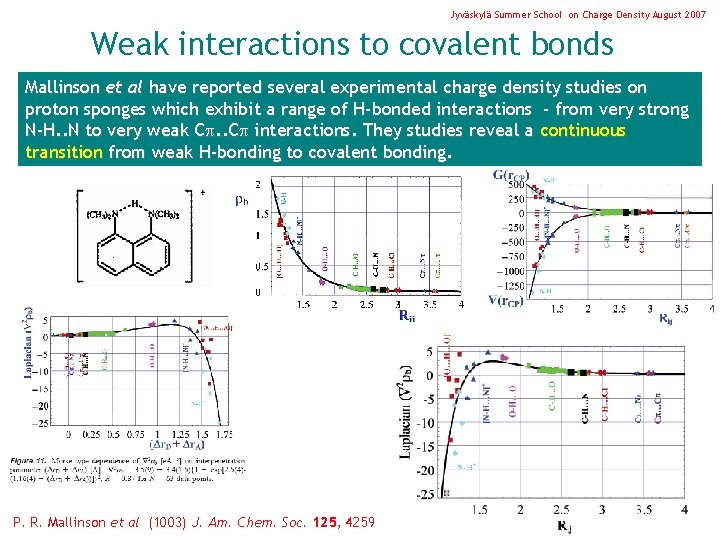
Jyväskylä Summer School on Charge Density August 2007 Weak interactions to covalent bonds Mallinson et al have reported several experimental charge density studies on proton sponges which exhibit a range of H-bonded interactions - from very strong N-H. . N to very weak C. . C interactions. They studies reveal a continuous transition from weak H-bonding to covalent bonding. P. R. Mallinson et al (1003) J. Am. Chem. Soc. 125, 4259

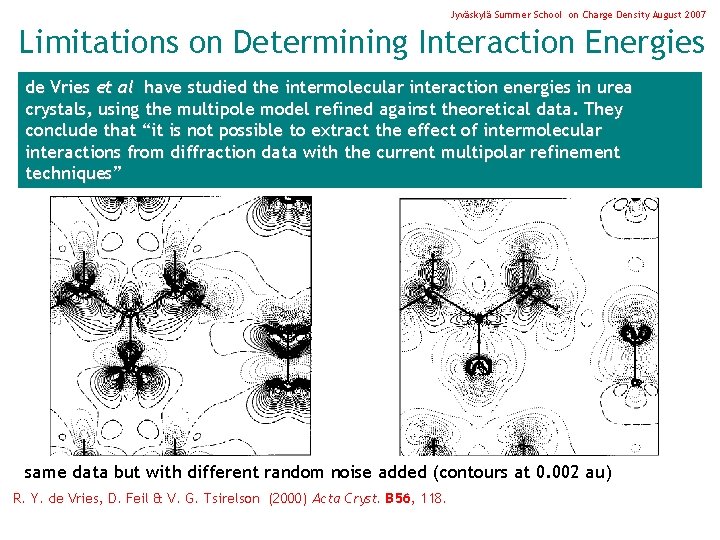
Jyväskylä Summer School on Charge Density August 2007 Limitations on Determining Interaction Energies de Vries et al have studied the intermolecular interaction energies in urea crystals, using the multipole model refined against theoretical data. They conclude that “it is not possible to extract the effect of intermolecular interactions from diffraction data with the current multipolar refinement techniques” same data but with different random noise added (contours at 0. 002 au) R. Y. de Vries, D. Feil & V. G. Tsirelson (2000) Acta Cryst. B 56, 118.

Jyväskylä Summer School on Charge Density August 2007 A Note of Caution “… for H…O interactions, critical point densities bcp and their distance dependence are closely similar to those calculated for the overlap of the unperturbed atomic charge densities (the promolecule electron density) so that great care in the interpretation of experimental and theoretical intermolecular charge densities is called for. ” J. Dunitz & A. Gavezzotti (2005) “Molecular Recognition in Organic Crystals – Directed Intermolecular Bonds or Nonlocalized Bonding ? ” Angew. Chem. Int. Ed. , 44, 1766. Review chapters on AIM concepts and H-bonding in books : "Hydrogen Bonding - New Insights" Ed A. J. Grabowski (2006) Springer, Dordrecht. "The Quantum Theory of Atoms in Molecules" Ed. C. E. Matta & R. Boyd (2007), Wiley-VCH, Weinheim.

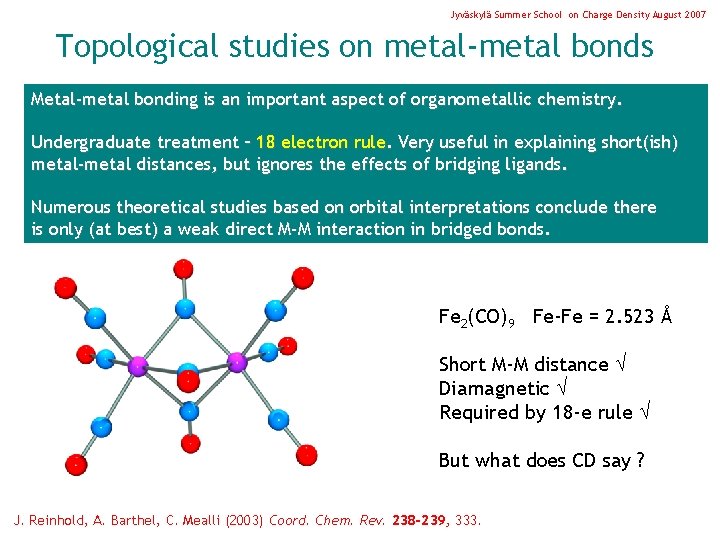
Jyväskylä Summer School on Charge Density August 2007 Topological studies on metal-metal bonds Metal-metal bonding is an important aspect of organometallic chemistry. Undergraduate treatment – 18 electron rule. Very useful in explaining short(ish) metal-metal distances, but ignores the effects of bridging ligands. Numerous theoretical studies based on orbital interpretations conclude there is only (at best) a weak direct M-M interaction in bridged bonds. Fe 2(CO)9 Fe-Fe = 2. 523 Å Short M-M distance Diamagnetic Required by 18 -e rule But what does CD say ? J. Reinhold, A. Barthel, C. Mealli (2003) Coord. Chem. Rev. 238 -239, 333.

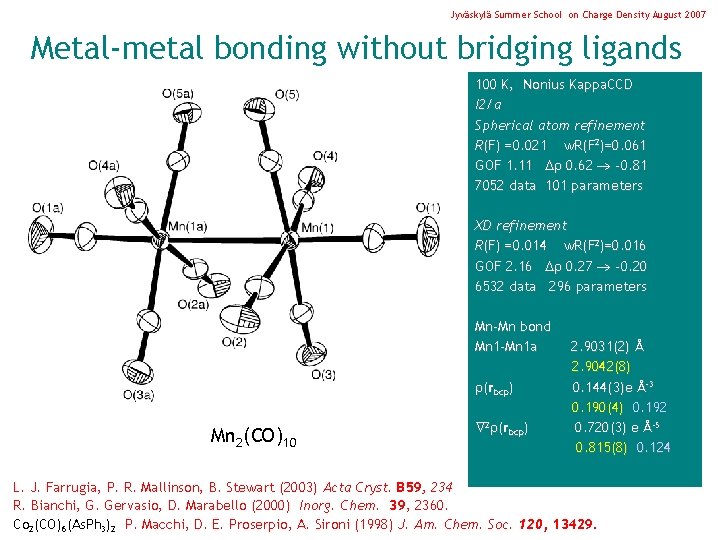
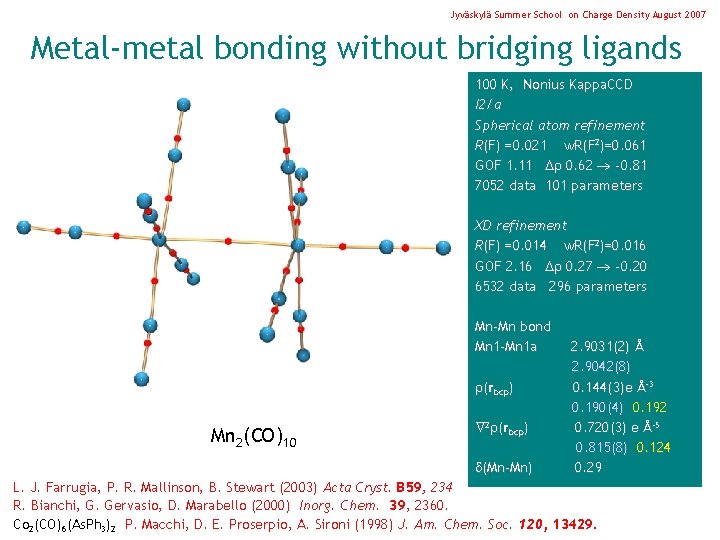
Jyväskylä Summer School on Charge Density August 2007 Metal-metal bonding without bridging ligands 100 K, Nonius Kappa. CCD I 2/a Spherical atom refinement R(F) =0. 021 w. R(F 2)=0. 061 GOF 1. 11 0. 62 -0. 81 7052 data 101 parameters XD refinement R(F) =0. 014 w. R(F 2)=0. 016 GOF 2. 16 0. 27 -0. 20 6532 data 296 parameters Mn-Mn bond Mn 1 -Mn 1 a (rbcp) Mn 2(CO)10 2 (rbcp) 2. 9031(2) Å 2. 9042(8) 0. 144(3)e Å-3 0. 190(4) 0. 192 0. 720(3) e Å-5 0. 815(8) 0. 124 L. J. Farrugia, P. R. Mallinson, B. Stewart (2003) Acta Cryst. B 59, 234 R. Bianchi, G. Gervasio, D. Marabello (2000) Inorg. Chem. 39, 2360. Co 2(CO)6(As. Ph 3)2 P. Macchi, D. E. Proserpio, A. Sironi (1998) J. Am. Chem. Soc. 120, 13429.

Jyväskylä Summer School on Charge Density August 2007 Metal-metal bonding without bridging ligands 100 K, Nonius Kappa. CCD I 2/a Spherical atom refinement R(F) =0. 021 w. R(F 2)=0. 061 GOF 1. 11 0. 62 -0. 81 7052 data 101 parameters XD refinement R(F) =0. 014 w. R(F 2)=0. 016 GOF 2. 16 0. 27 -0. 20 6532 data 296 parameters Mn-Mn bond Mn 1 -Mn 1 a (rbcp) Mn 2(CO)10 2 (rbcp) (Mn-Mn) 2. 9031(2) Å 2. 9042(8) 0. 144(3)e Å-3 0. 190(4) 0. 192 0. 720(3) e Å-5 0. 815(8) 0. 124 0. 29 L. J. Farrugia, P. R. Mallinson, B. Stewart (2003) Acta Cryst. B 59, 234 R. Bianchi, G. Gervasio, D. Marabello (2000) Inorg. Chem. 39, 2360. Co 2(CO)6(As. Ph 3)2 P. Macchi, D. E. Proserpio, A. Sironi (1998) J. Am. Chem. Soc. 120, 13429.

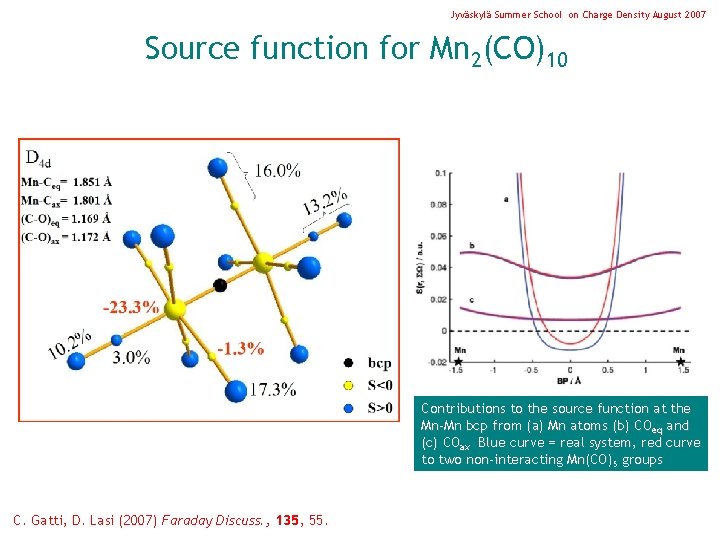
Jyväskylä Summer School on Charge Density August 2007 Source function for Mn 2(CO)10 Contributions to the source function at the Mn-Mn bcp from (a) Mn atoms (b) COeq and (c) COax Blue curve = real system, red curve to two non-interacting Mn(CO)5 groups C. Gatti, D. Lasi (2007) Faraday Discuss. , 135, 55.

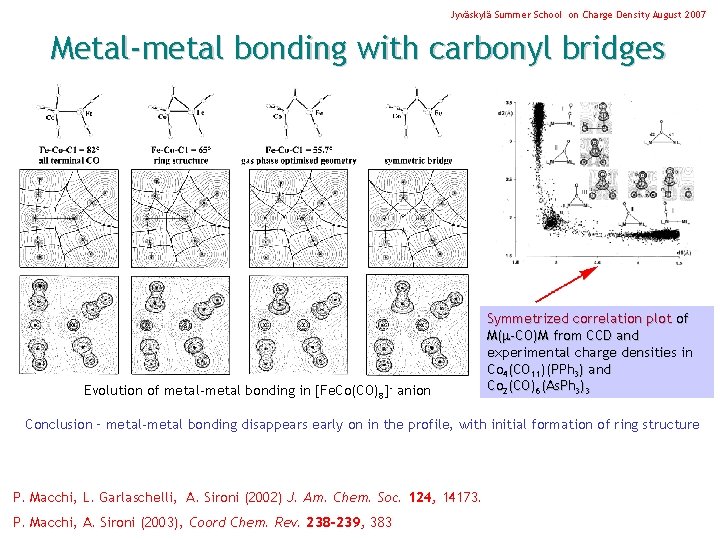
Jyväskylä Summer School on Charge Density August 2007 Metal-metal bonding with carbonyl bridges Evolution of metal-metal bonding in [Fe. Co(CO)8]- anion Symmetrized correlation plot of M( -CO)M from CCD and experimental charge densities in Co 4(CO 11)(PPh 3) and Co 2(CO)6(As. Ph 3)3 Conclusion – metal-metal bonding disappears early on in the profile, with initial formation of ring structure P. Macchi, L. Garlaschelli, A. Sironi (2002) J. Am. Chem. Soc. 124, 14173. P. Macchi, A. Sironi (2003), Coord Chem. Rev. 238 -239, 383

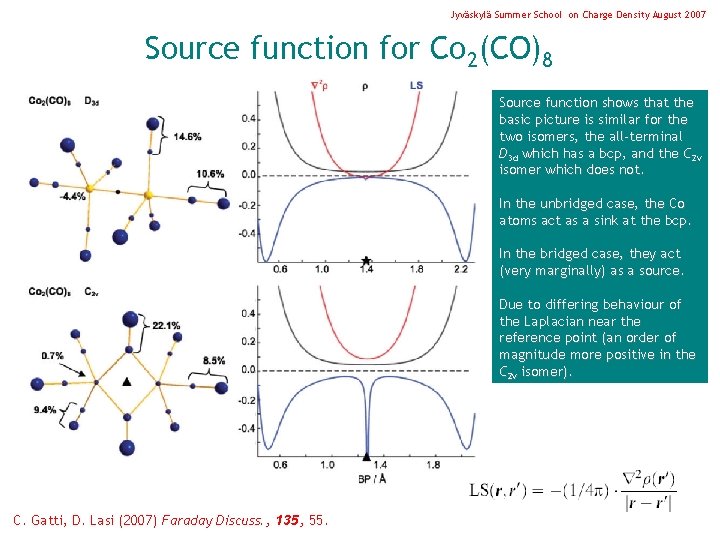
Jyväskylä Summer School on Charge Density August 2007 Source function for Co 2(CO)8 Source function shows that the basic picture is similar for the two isomers, the all-terminal D 3 d which has a bcp, and the C 2 v isomer which does not. In the unbridged case, the Co atoms act as a sink at the bcp. In the bridged case, they act (very marginally) as a source. Due to differing behaviour of the Laplacian near the reference point (an order of magnitude more positive in the C 2 v isomer). C. Gatti, D. Lasi (2007) Faraday Discuss. , 135, 55.

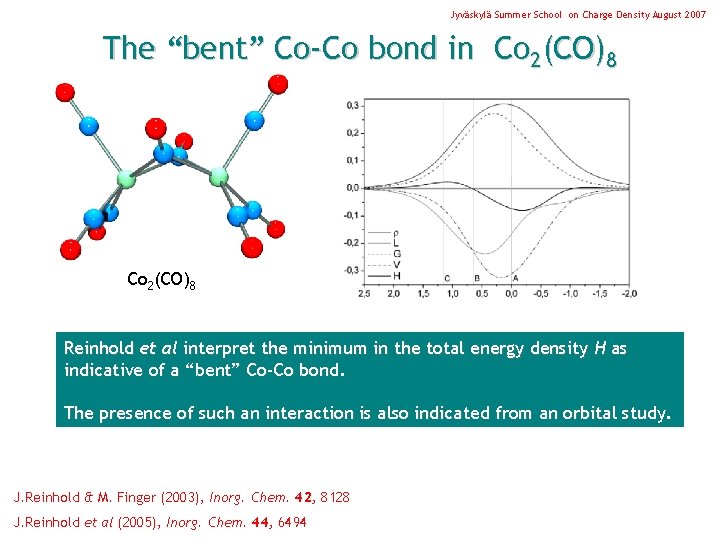
Jyväskylä Summer School on Charge Density August 2007 The “bent” Co-Co bond in Co 2(CO)8 Reinhold et al interpret the minimum in the total energy density H as indicative of a “bent” Co-Co bond. The presence of such an interaction is also indicated from an orbital study. J. Reinhold & M. Finger (2003), Inorg. Chem. 42, 8128 J. Reinhold et al (2005), Inorg. Chem. 44, 6494

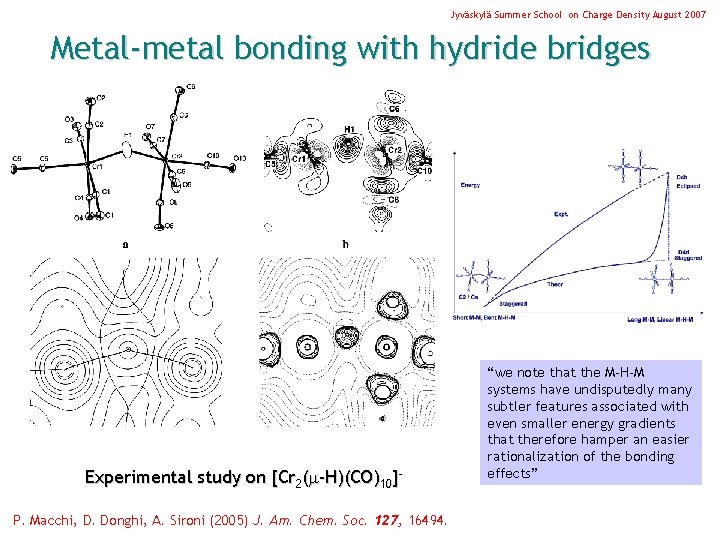
Jyväskylä Summer School on Charge Density August 2007 Metal-metal bonding with hydride bridges Experimental study on [Cr 2( -H)(CO)10]P. Macchi, D. Donghi, A. Sironi (2005) J. Am. Chem. Soc. 127, 16494. “we note that the M-H-M systems have undisputedly many subtler features associated with even smaller energy gradients that therefore hamper an easier rationalization of the bonding effects”

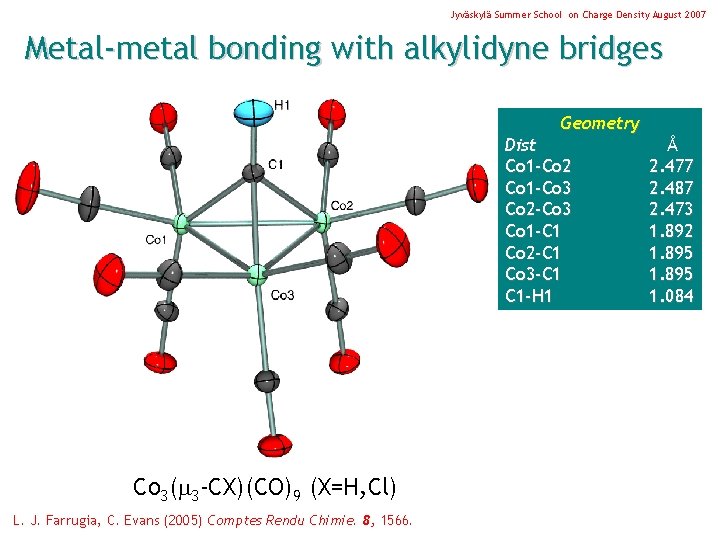
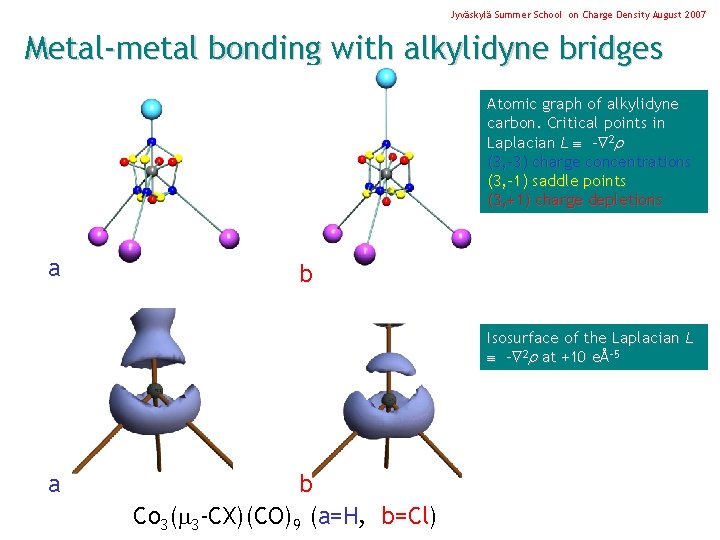
Jyväskylä Summer School on Charge Density August 2007 Metal-metal bonding with alkylidyne bridges Geometry Dist Co 1 -Co 2 Co 1 -Co 3 Co 2 -Co 3 Co 1 -C 1 Co 2 -C 1 Co 3 -C 1 C 1 -H 1 Co 3( 3 -CX)(CO)9 (X=H, Cl) L. J. Farrugia, C. Evans (2005) Comptes Rendu Chimie. 8, 1566. Å 2. 477 2. 487 2. 473 1. 892 1. 895 1. 084

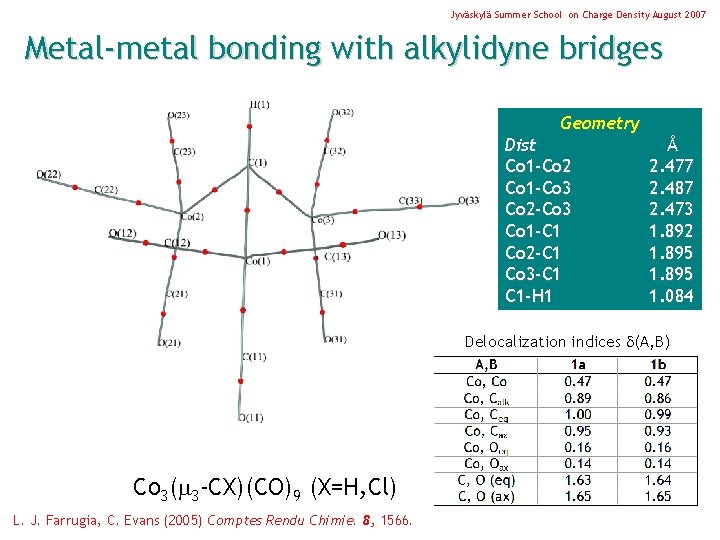
Jyväskylä Summer School on Charge Density August 2007 Metal-metal bonding with alkylidyne bridges Geometry Dist Co 1 -Co 2 Co 1 -Co 3 Co 2 -Co 3 Co 1 -C 1 Co 2 -C 1 Co 3 -C 1 C 1 -H 1 Å 2. 477 2. 487 2. 473 1. 892 1. 895 1. 084 Delocalization indices (A, B) Co 3( 3 -CX)(CO)9 (X=H, Cl) L. J. Farrugia, C. Evans (2005) Comptes Rendu Chimie. 8, 1566.

Jyväskylä Summer School on Charge Density August 2007 Metal-metal bonding with alkylidyne bridges Atomic graph of alkylidyne carbon. Critical points in Laplacian L - 2 (3, -3) charge concentrations (3, -1) saddle points (3, +1) charge depletions a b Isosurface of the Laplacian L - 2 at +10 eÅ-5 a b Co 3( 3 -CX)(CO)9 (a=H, b=Cl)

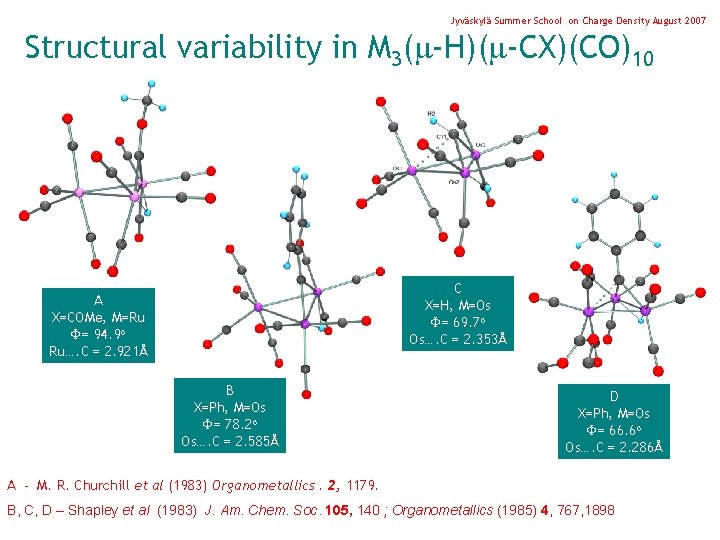
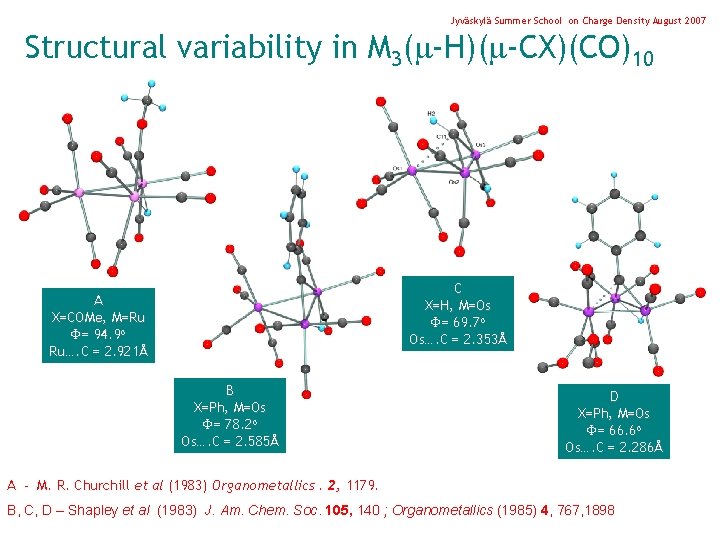
Jyväskylä Summer School on Charge Density August 2007 Structural variability in M 3( -H)( -CX)(CO)10 C X=H, M=Os = 69. 7 o Os…. C = 2. 353Å A X=COMe, M=Ru = 94. 9 o Ru…. C = 2. 921Å B X=Ph, M=Os = 78. 2 o Os…. C = 2. 585Å D X=Ph, M=Os = 66. 6 o Os…. C = 2. 286Å A - M. R. Churchill et al (1983) Organometallics. 2, 1179. B, C, D – Shapley et al (1983) J. Am. Chem. Soc. 105, 140 ; Organometallics (1985) 4, 767, 1898

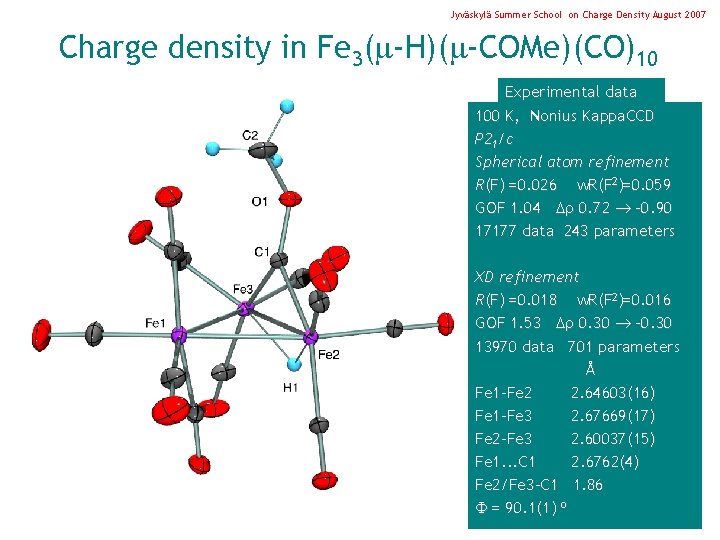
Jyväskylä Summer School on Charge Density August 2007 Charge density in Fe 3( -H)( -COMe)(CO)10 Experimental data 100 K, Nonius Kappa. CCD P 21/c Spherical atom refinement R(F) =0. 026 w. R(F 2)=0. 059 GOF 1. 04 0. 72 -0. 90 17177 data 243 parameters XD refinement R(F) =0. 018 w. R(F 2)=0. 016 GOF 1. 53 0. 30 -0. 30 13970 data 701 parameters Å Fe 1 -Fe 2 2. 64603(16) Fe 1 -Fe 3 2. 67669(17) Fe 2 -Fe 3 2. 60037(15) Fe 1. . . C 1 2. 6762(4) Fe 2/Fe 3 -C 1 1. 86 = 90. 1(1) o

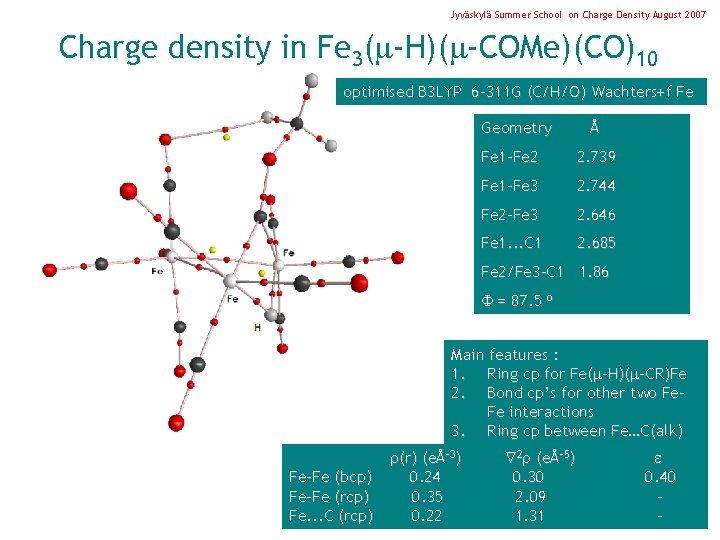
Jyväskylä Summer School on Charge Density August 2007 Charge density in Fe 3( -H)( -COMe)(CO)10 optimised B 3 LYP 6 -311 G (C/H/O) Wachters+f Fe Geometry Å Fe 1 -Fe 2 2. 739 Fe 1 -Fe 3 2. 744 Fe 2 -Fe 3 2. 646 Fe 1. . . C 1 2. 685 Fe 2/Fe 3 -C 1 1. 86 = 87. 5 o Main features : 1. Ring cp for Fe( -H)( -CR)Fe 2. Bond cp’s for other two Fe. Fe interactions 3. Ring cp between Fe…C(alk) Fe-Fe (bcp) Fe-Fe (rcp) Fe. . . C (rcp) (r) (eÅ-3) 0. 24 0. 35 0. 22 2 (eÅ-5) 0. 30 2. 09 1. 31 0. 40 -

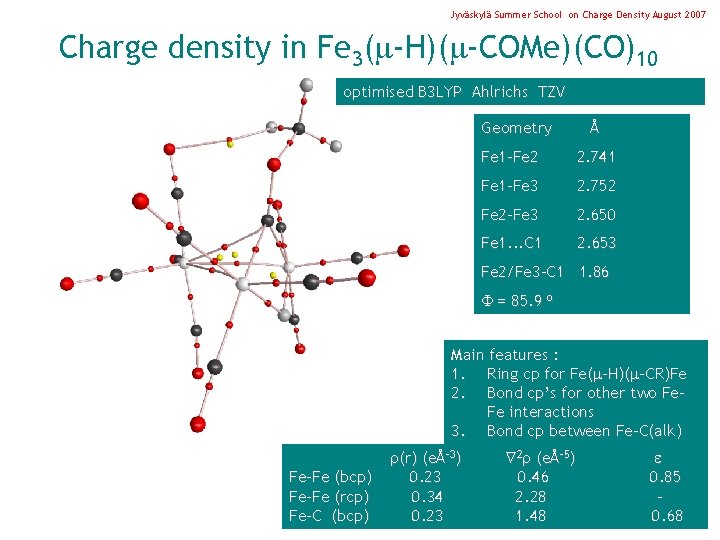
Jyväskylä Summer School on Charge Density August 2007 Charge density in Fe 3( -H)( -COMe)(CO)10 optimised B 3 LYP Ahlrichs TZV Geometry Å Fe 1 -Fe 2 2. 741 Fe 1 -Fe 3 2. 752 Fe 2 -Fe 3 2. 650 Fe 1. . . C 1 2. 653 Fe 2/Fe 3 -C 1 1. 86 = 85. 9 o Main features : 1. Ring cp for Fe( -H)( -CR)Fe 2. Bond cp’s for other two Fe. Fe interactions 3. Bond cp between Fe-C(alk) Fe-Fe (bcp) Fe-Fe (rcp) Fe-C (bcp) (r) (eÅ-3) 0. 23 0. 34 0. 23 2 (eÅ-5) 0. 46 2. 28 1. 48 0. 85 0. 68

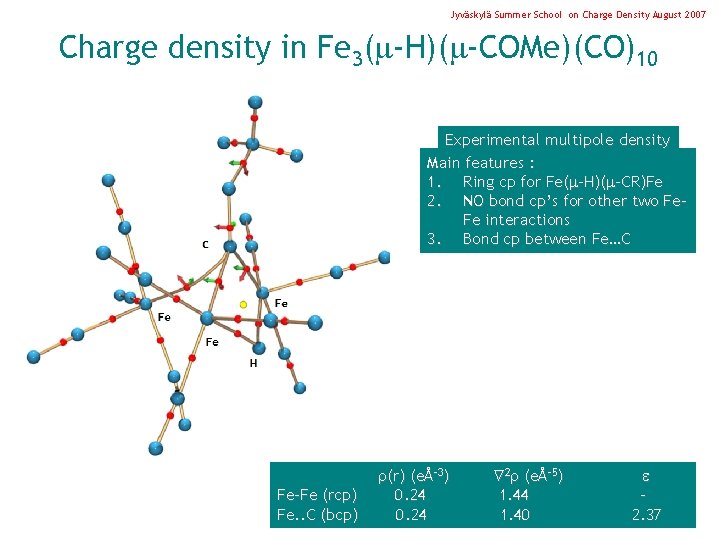
Jyväskylä Summer School on Charge Density August 2007 Charge density in Fe 3( -H)( -COMe)(CO)10 Experimental multipole density Main features : 1. Ring cp for Fe( -H)( -CR)Fe 2. NO bond cp’s for other two Fe. Fe interactions 3. Bond cp between Fe…C Fe-Fe (rcp) Fe. . C (bcp) (r) (eÅ-3) 0. 24 2 (eÅ-5) 1. 44 1. 40 2. 37

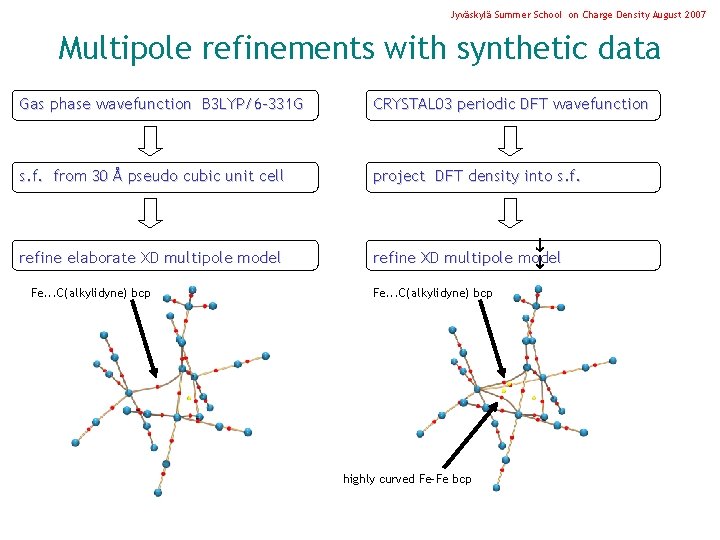
Jyväskylä Summer School on Charge Density August 2007 Multipole refinements with synthetic data Gas phase wavefunction B 3 LYP/6 -331 G CRYSTAL 03 periodic DFT wavefunction s. f. from 30 Å pseudo cubic unit cell project DFT density into s. f. refine elaborate XD multipole model refine XD multipole model Fe. . . C(alkylidyne) bcp highly curved Fe-Fe bcp

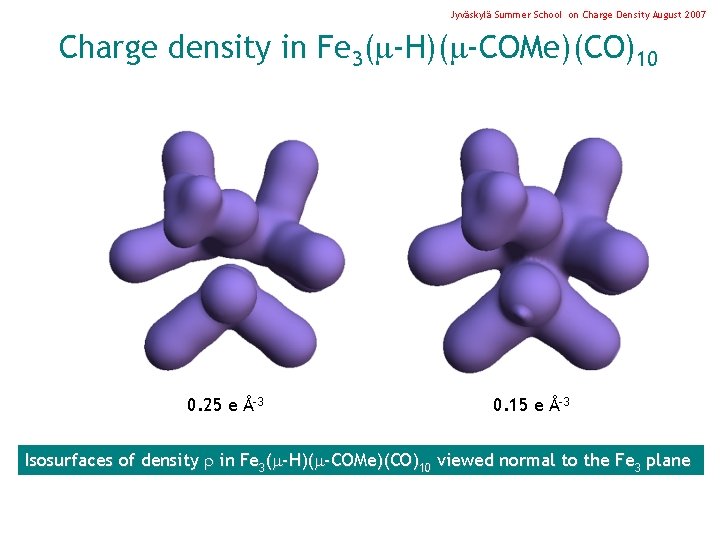
Jyväskylä Summer School on Charge Density August 2007 Charge density in Fe 3( -H)( -COMe)(CO)10 0. 25 e Å-3 0. 15 e Å-3 Isosurfaces of density in Fe 3( -H)( -COMe)(CO)10 viewed normal to the Fe 3 plane

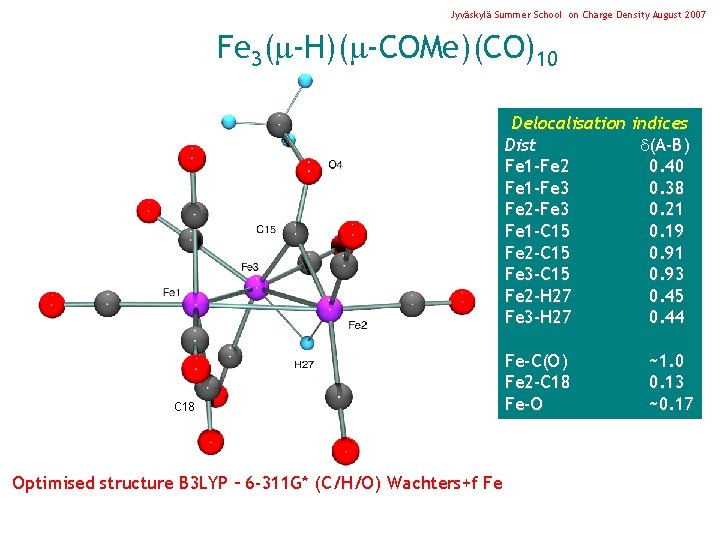
Jyväskylä Summer School on Charge Density August 2007 Fe 3( -H)( -COMe)(CO)10 Delocalisation indices Dist (A-B) Fe 1 -Fe 2 0. 40 Fe 1 -Fe 3 0. 38 Fe 2 -Fe 3 0. 21 Fe 1 -C 15 0. 19 Fe 2 -C 15 0. 91 Fe 3 -C 15 0. 93 Fe 2 -H 27 0. 45 Fe 3 -H 27 0. 44 C 18 Optimised structure B 3 LYP – 6 -311 G* (C/H/O) Wachters+f Fe Fe-C(O) Fe 2 -C 18 Fe-O ~1. 0 0. 13 ~0. 17

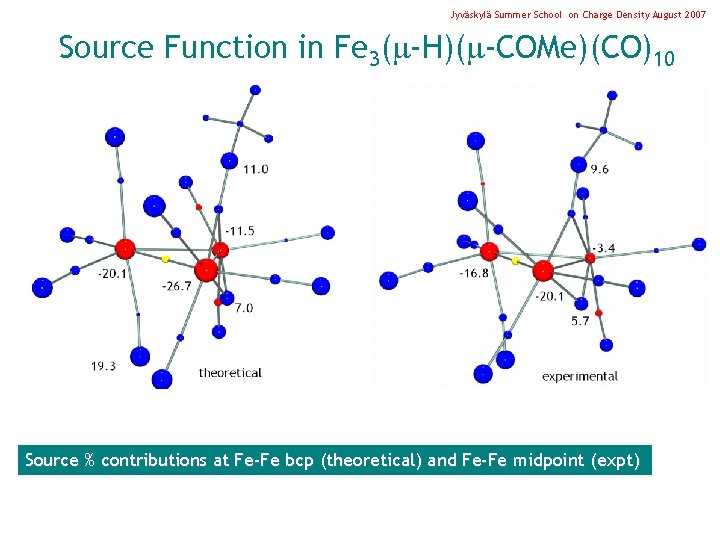
Jyväskylä Summer School on Charge Density August 2007 Source Function in Fe 3( -H)( -COMe)(CO)10 Source % contributions at Fe-Fe bcp (theoretical) and Fe-Fe midpoint (expt)

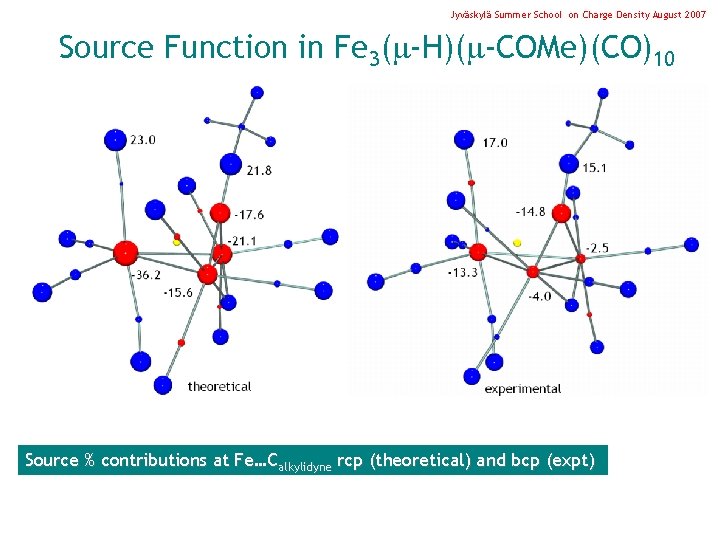
Jyväskylä Summer School on Charge Density August 2007 Source Function in Fe 3( -H)( -COMe)(CO)10 Source % contributions at Fe…Calkylidyne rcp (theoretical) and bcp (expt)

Jyväskylä Summer School on Charge Density August 2007 Structural variability in M 3( -H)( -CX)(CO)10 C X=H, M=Os = 69. 7 o Os…. C = 2. 353Å A X=COMe, M=Ru = 94. 9 o Ru…. C = 2. 921Å B X=Ph, M=Os = 78. 2 o Os…. C = 2. 585Å D X=Ph, M=Os = 66. 6 o Os…. C = 2. 286Å A - M. R. Churchill et al (1983) Organometallics. 2, 1179. B, C, D – Shapley et al (1983) J. Am. Chem. Soc. 105, 140 ; Organometallics (1985) 4, 767, 1898

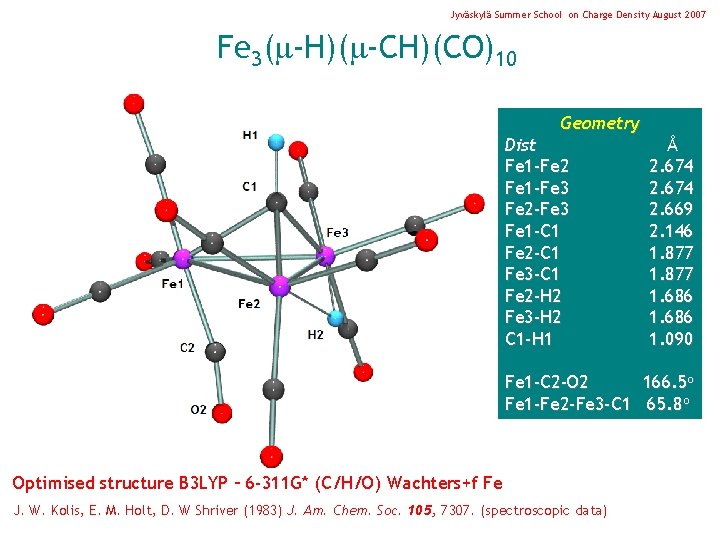
Jyväskylä Summer School on Charge Density August 2007 Fe 3( -H)( -CH)(CO)10 Geometry Dist Fe 1 -Fe 2 Fe 1 -Fe 3 Fe 2 -Fe 3 Fe 1 -C 1 Fe 2 -C 1 Fe 3 -C 1 Fe 2 -H 2 Fe 3 -H 2 C 1 -H 1 Å 2. 674 2. 669 2. 146 1. 877 1. 686 1. 090 Fe 1 -C 2 -O 2 166. 5 o Fe 1 -Fe 2 -Fe 3 -C 1 65. 8 o Optimised structure B 3 LYP – 6 -311 G* (C/H/O) Wachters+f Fe J. W. Kolis, E. M. Holt, D. W Shriver (1983) J. Am. Chem. Soc. 105, 7307. (spectroscopic data)

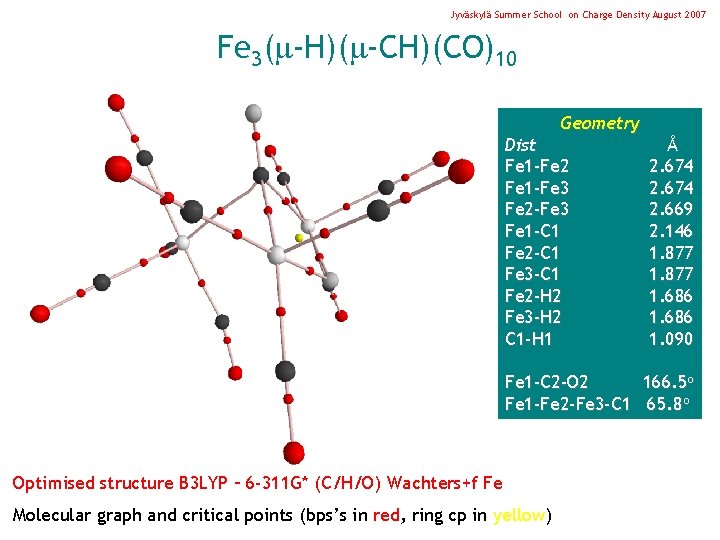
Jyväskylä Summer School on Charge Density August 2007 Fe 3( -H)( -CH)(CO)10 Geometry Dist Fe 1 -Fe 2 Fe 1 -Fe 3 Fe 2 -Fe 3 Fe 1 -C 1 Fe 2 -C 1 Fe 3 -C 1 Fe 2 -H 2 Fe 3 -H 2 C 1 -H 1 Å 2. 674 2. 669 2. 146 1. 877 1. 686 1. 090 Fe 1 -C 2 -O 2 166. 5 o Fe 1 -Fe 2 -Fe 3 -C 1 65. 8 o Optimised structure B 3 LYP – 6 -311 G* (C/H/O) Wachters+f Fe Molecular graph and critical points (bps’s in red, ring cp in yellow)