Journal Review Fecal Microbiota Transplantation SNUBH F JH

소화기내과 집담회 Journal Review: Fecal Microbiota Transplantation SNUBH F. JH CHO / Prof. DH LEE 2018. 12. 13

Italian Neurological Society

Introduction PD and intestinal flora • • • Before being diagnosed with PD, – many patients reported constipation as one of the initial gastrointestinal symptoms – Constipation is common, 70 -80% in PD PD causes other gastrointestinal symptoms, including abdominal pain, bloating, and incomplete defecation Microbial treatment – Probiotics; effectively – Fecal microbiota transplantation (FMT); possibly in PD
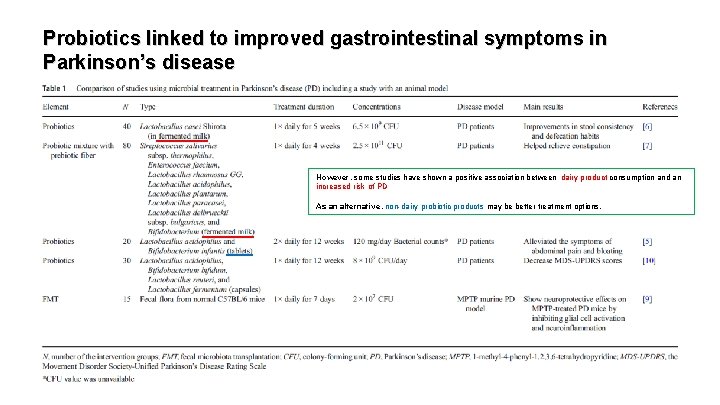
Probiotics linked to improved gastrointestinal symptoms in Parkinson’s disease However, some studies have shown a positive association between dairy product consumption and an increased risk of PD As an alternative, non-dairy probiotic products may be better treatment options.

• The precise underlying mechanisms of probiotic in improving gastrointestinal symptoms in PD are not fully clarified – Probiotics can increase gut motility as they directly stimulate intestinal smooth muscle cells • Irritable bowel syndrome (IBS)-like bowel symptoms; 24. 3% of PD – linked to a lower abundance of Prevotella and Bifidobacterium species

Probiotics improve gastrointestinal symptoms and more in Parkinson’s disease Delayed gastric emptying • • • Common in PD Negatively affect the absorption of some oral PD medications like levodopa Probiotic Lactobacillus reuteri DSM 17938 – enhance gastric emptying in infants – suggesting that this therapy may be effective in PD

Decreased L-dopa absorption and increased motor function fluctuations • • • Occur in PD with Helicobacter pylori (HP) infection Reversed by HP eradication Probiotic supplementation contributes to HP eradication – and it reduces the severity of antibiotic-induced adverse effects

Laxatives • • • May decrease the worsening of rigidity in PD Possible mechanical perturbations induced by laxatives May impact the intestinal microflora • • Probiotic use can decrease MDS-UPDRS scores in PD However, its effect on the pathological changes in the brain is still unknown
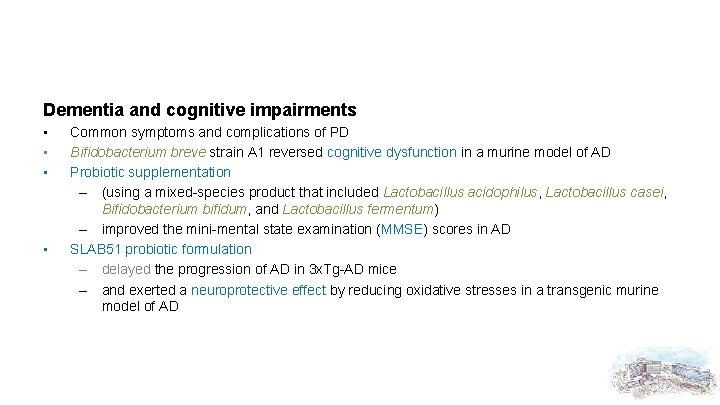
Dementia and cognitive impairments • • Common symptoms and complications of PD Bifidobacterium breve strain A 1 reversed cognitive dysfunction in a murine model of AD Probiotic supplementation – (using a mixed-species product that included Lactobacillus acidophilus, Lactobacillus casei, Bifidobacterium bifidum, and Lactobacillus fermentum) – improved the mini-mental state examination (MMSE) scores in AD SLAB 51 probiotic formulation – delayed the progression of AD in 3 x. Tg-AD mice – and exerted a neuroprotective effect by reducing oxidative stresses in a transgenic murine model of AD

Depression and anxiety • • • Common in PD-associated dementia Low serotonin levels Lactobacillus plantarum PS 128 can upregulate dopamine and serotonin levels in the striatum of the germ-free mice Bifidobacterium longum NCC 3001 can decrease the depression scores in IBS Lactobacillus helveticus and Bifidobacterium longum can decrease the Beck Depression Inventory scores in MDD
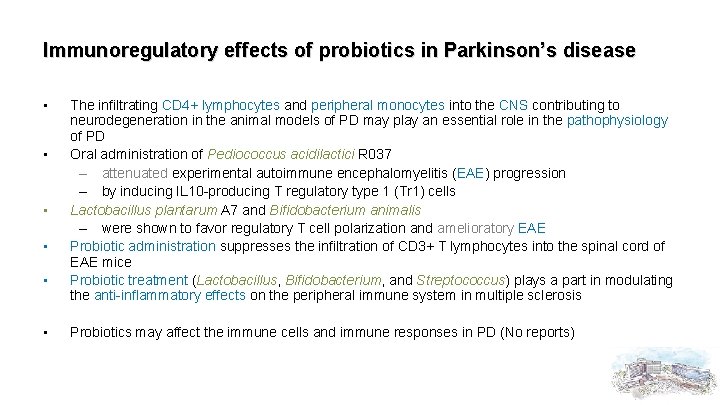
Immunoregulatory effects of probiotics in Parkinson’s disease • • • The infiltrating CD 4+ lymphocytes and peripheral monocytes into the CNS contributing to neurodegeneration in the animal models of PD may play an essential role in the pathophysiology of PD Oral administration of Pediococcus acidilactici R 037 – attenuated experimental autoimmune encephalomyelitis (EAE) progression – by inducing IL 10 -producing T regulatory type 1 (Tr 1) cells Lactobacillus plantarum A 7 and Bifidobacterium animalis – were shown to favor regulatory T cell polarization and amelioratory EAE Probiotic administration suppresses the infiltration of CD 3+ T lymphocytes into the spinal cord of EAE mice Probiotic treatment (Lactobacillus, Bifidobacterium, and Streptococcus) plays a part in modulating the anti-inflammatory effects on the peripheral immune system in multiple sclerosis Probiotics may affect the immune cells and immune responses in PD (No reports)

Cause or effect? Link between intestinal microflora changes and Parkinson’s disease • • • Alteration of fecal microbiota precedes the onset of brain pathology in rotenone-treated murine models of PD The intestinal flora changes as PD progresses, which directly correlates with the clinical features of the disease These studies suggested that alterations of intestinal flora may not just be a phenomenon of PD

• Alpha-synuclein-overexpressing mice (treated with gut microbes from PD patients) showed aggravated motor deficits • Intestinal microbes and SCFA regulate microglial cell in CNS • 1 -Methyl-4 -phenyl-1, 2, 3, 6 -tetrahydropyridine (MPTP)-treated PD mice – exhibited an increased presence of Proteus mirabilis in the feces. – In addition, oral co-treatment with Proteus mirabilis aggravated the loss of dopaminergic neurons and motor impairment, – along with activation of microglia in the substantia nigra and striatum in MPTP-treated mice
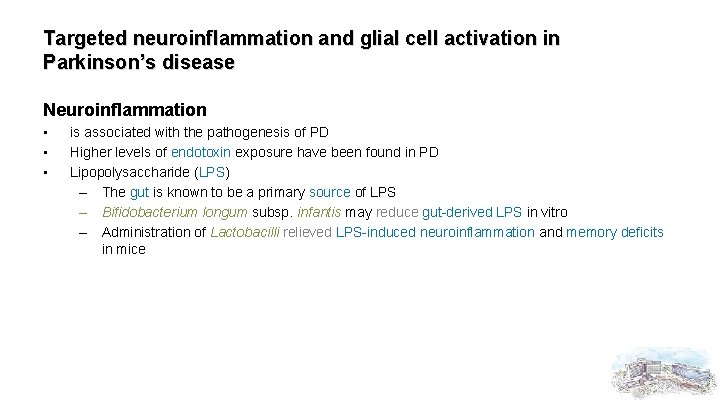
Targeted neuroinflammation and glial cell activation in Parkinson’s disease Neuroinflammation • • • is associated with the pathogenesis of PD Higher levels of endotoxin exposure have been found in PD Lipopolysaccharide (LPS) – The gut is known to be a primary source of LPS – Bifidobacterium longum subsp. infantis may reduce gut-derived LPS in vitro – Administration of Lactobacilli relieved LPS-induced neuroinflammation and memory deficits in mice

Mitochondrial dysfunction • • • is another condition in PD Prebiotics (xylooligosaccharide), probiotics (Lactobacillus paracasei HII 01), and synbiotics can reduce hippocampal microglia activation and cognitive deficits, as well as attenuate the dysfunction of brain mitochondrion, in obese insulin-resistant rats
Role of fecal microbiota transplantation in Parkinson’s disease FMT is a new treatment option • • Initial success in the treatment of C. difficile infection and IBS Gut microbial dysbiosis and increased fecal SCFA concentrations have been observed in MPTPtreated PD mice • FMT from normal mice has shown neuroprotective effects on MPTP-treated PD mice – by inhibiting glial cell activation and neuroinflammation It has been reported that SCFA can exacerbate motor symptoms in α-synuclein PD mice. – However, fecal SCFA concentrations decreased following FMT treatment can relieve constipation symptoms in patients with slow transit constipation • •

Conclusions • In conclusion, microbial therapies may be beneficial for PD patients suffering from gastrointestinal disorders • The composition and diversity of the intestinal microflora and baseline symptoms vary between people, so the microbial treatment for PD may be individualized.

2018 IF : 2. 037
Introduction Irritable bowel syndrome (IBS) • • • Intestinal microbiota, features of lower diversity and higher instability compared to healthy controls The concept of gut microbial dysbiosis in IBS Management of IBS – use of antibiotics or probiotics – dietary approaches with diets of reduced fermentable oligo-, di-, monosaccharides, and polyols contents – Fecal microbiota transfer (FMT); another approach
Fecal Microbiota Transplantation • • • However, remains controversial for several clinical conditions other than recurrent Clostridium difficile infections Recently, a first randomized trial of FMT in IBS has been reported to show a favorable outcome of FMT on IBS severity; – but, limited, due to a lack of data on microbiome changes after FMT We investigated its safety and clinical responses and studied FMT-associated gut microbiota changes.

Materials and Methods Study Cohort • • • Recruitment: – fulfilling the DGVS/DGNM German Guidelines for IBS and Rome III criteria – with a Symptom history longer then 2 years that were refractory or not sufficiently resolved after dietary changes, anti- or probiotic treatments, spasmolytic drugs, or other IBS medication. Exclusion criteria: – pregnancy, lactation – systemic disease, infections – antibiotic treatments in the last 3 months – major changes in lactose, fructose and sorbitol diets in the last 3 months – and mental illness Screened 27 Final 13

Study Outline • • • 2 consecutive FMT from one healthy universal donor Outcome: – Symptoms – Qo. L parameters – Microbiota profiles Monitor: – baseline (week -4; before bowel preparation) – 1, 5, 9, 13 weeks after FMT

Administration of Donor Material • • • Donor: healthy male, 28 age, BMI 22 No relationship with patients or study staff Screening: – Questionnaire; • infectious, neoplastic and mental risk factors – Fecal samples tested; • parasites (eggs, cysts and larvae), enteropathogenic bacteria (C. difficile [GDH assay], Salmonella spp. , Campylobacter spp. , Yersinia spp. , enteropathogenic E. coli, S. aureus, H. pylori), and viruses (Adenovirus, Rotavirus, Norovirus) – Serological screening; • HIV-1, 2, hepatitis A, B, C, T. pallidum
![Donor material • According to the protocol of Hamilton et al [25] • Feces Donor material • According to the protocol of Hamilton et al [25] • Feces](http://slidetodoc.com/presentation_image_h2/e4c6ea1369782704b1edd0953e3eb6fe/image-24.jpg)
Donor material • According to the protocol of Hamilton et al [25] • Feces weighing 50 m. L were frozen at – 80 ° C as 20% glycerol suspensions in PBS • Slowly defrosted and heated in a water bath at 37 ° C for 30 -60 min before application • In total, 7 donations [25] Hamilton MJ, et al. Standardized frozen preparation for transplantation of fecal microbiota for recurrent Clostridium difficile infection. Am J Gastroenterol 2012; 107: 761 -7.

FMT procedures • • • pretreatment with 800 mg (2 × 400 mg) rifaximin as a non-absorbable, broad-spectrum antibiotic drug for 21 days to increase engraftment of donor microbiota [26] On day 22, patients underwent bowel lavage using macrogol First transfer; – • Second transfer; – – • colonoscopic injection into the terminal ileum and cecum, 100 -150 m. L of the donor fecal suspension (corresponding to approximately 50 g stool) on the next day, via a rectal enema applying the same amount of donor fecal suspension After the enema, participants had to lay on their left side for about 30 min For 3 days after the transfers, patients received 2 mg loperamide to decrease bowel movements [26] Keshteli AH, et al. Pretreatment with antibiotics may enhance the efficacy of fecal microbiota transplantation in ulcerative colitis: a meta-analysis. Mucosal Immunol 2017; 10: 565– 66.
Analysis of Fecal Microbiota • • • -4 week (baseline) and 9 weeks after FMT 16 S ribosomal RNA (r. RNA) gene amplicon analyses, spanning the V 3 -V 4 hypervariable region Using a Mi. Seq system (Illumina, San Diego, CA, USA) Processed using the IMNGS pipeline Operational taxonomic units (OTUs); clustered at 97% sequence similarity using the RDP classifier, and only those with a relative abundance > 0. 5% in at least 1 sample were kept

Statistical Analysis • Alpha-diversity; – – • basis of species richness and Shannon effective counts using the LEf. Se algorithm (https: //bitbucket. org/nsegata/lefse) Beta-diversity; – – – basis of generalized Uni. Frac distances using t-SNE in the R package with generalized Uni. Frac distance measure (perplexity = 7, maximum iterations = 3, 000) also computed multidimensional scaling (MDS)

RESULTS
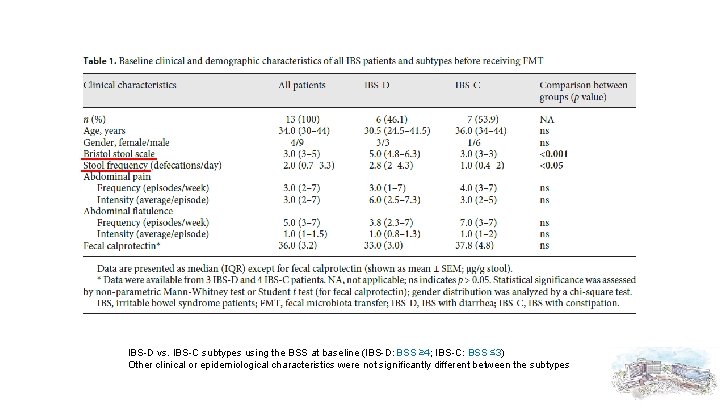
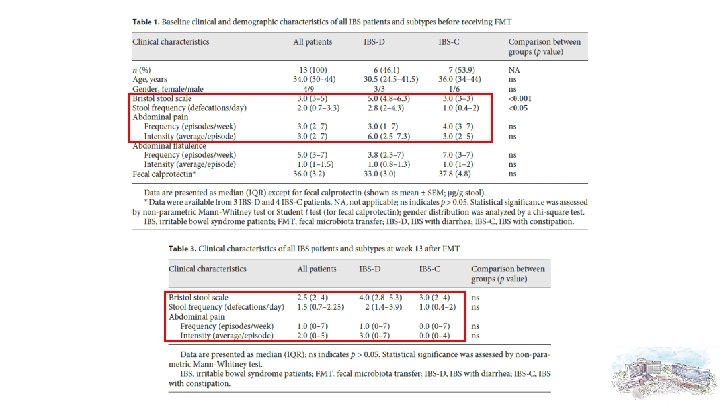
IBS-D vs. IBS-C subtypes using the BSS at baseline (IBS-D: BSS ≥ 4; IBS-C: BSS ≤ 3) Other clinical or epidemiological characteristics were not significantly different between the subtypes
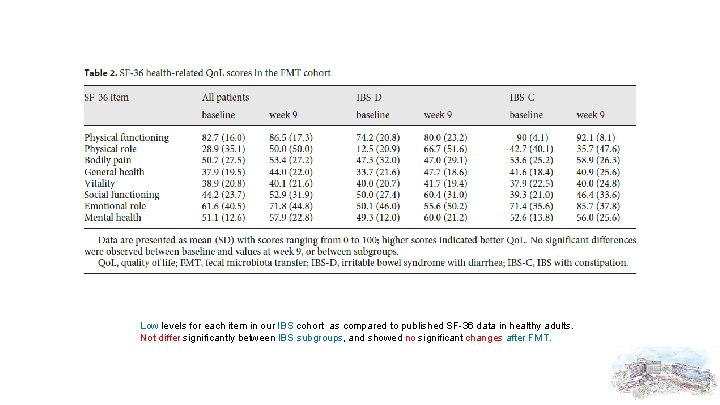
Low levels for each item in our IBS cohort as compared to published SF-36 data in healthy adults. Not differ significantly between IBS subgroups, and showed no significant changes after FMT.

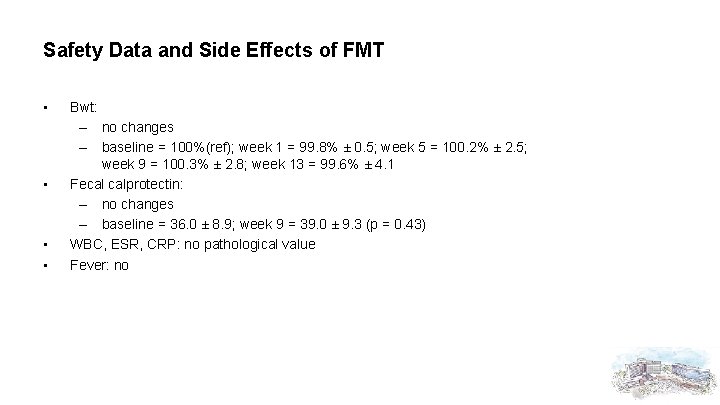
Safety Data and Side Effects of FMT • • Bwt: – no changes – baseline = 100%(ref); week 1 = 99. 8% ± 0. 5; week 5 = 100. 2% ± 2. 5; week 9 = 100. 3% ± 2. 8; week 13 = 99. 6% ± 4. 1 Fecal calprotectin: – no changes – baseline = 36. 0 ± 8. 9; week 9 = 39. 0 ± 9. 3 (p = 0. 43) WBC, ESR, CRP: no pathological value Fever: no
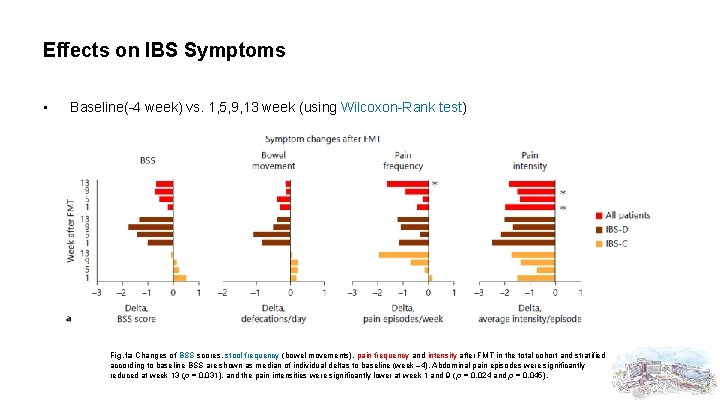
Effects on IBS Symptoms • Baseline(-4 week) vs. 1, 5, 9, 13 week (using Wilcoxon-Rank test) Fig. 1 a Changes of BSS scores, stool frequency (bowel movements), pain frequency and intensity after FMT in the total cohort and stratified according to baseline BSS are shown as median of individual deltas to baseline (week – 4). Abdominal pain episodes were significantly reduced at week 13 (p = 0. 031), and the pain intensities were significantly lower at week 1 and 9 ( p = 0. 024 and p = 0. 045).
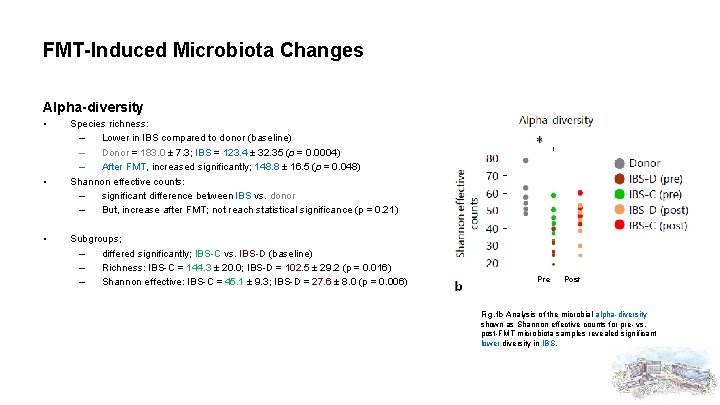
FMT-Induced Microbiota Changes Alpha-diversity • • • Species richness: – Lower in IBS compared to donor (baseline) – Donor = 183. 0 ± 7. 3; IBS = 123. 4 ± 32. 35 (p = 0. 0004) – After FMT, increased significantly; 148. 8 ± 16. 5 (p = 0. 048) Shannon effective counts: – significant difference between IBS vs. donor – But, increase after FMT; not reach statistical significance (p = 0. 21) Subgroups; – differed significantly; IBS-C vs. IBS-D (baseline) – Richness: IBS-C = 144. 3 ± 20. 0; IBS-D = 102. 5 ± 29. 2 (p = 0. 016) – Shannon effective: IBS-C = 45. 1 ± 9. 3; IBS-D = 27. 6 ± 8. 0 (p = 0. 006) Pre Post Fig. 1 b Analysis of the microbial alpha-diversity shown as Shannon effective counts for pre- vs. post-FMT microbiota samples revealed significant lower diversity in IBS.
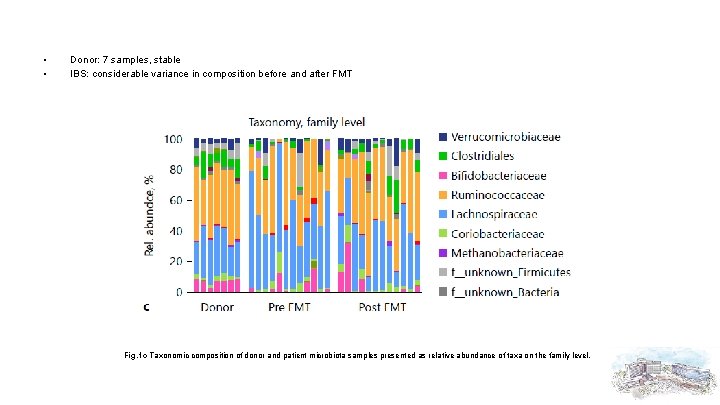
• • Donor: 7 samples, stable IBS: considerable variance in composition before and after FMT Fig. 1 c Taxonomic composition of donor and patient microbiota samples presented as relative abundance of taxa on the family level.
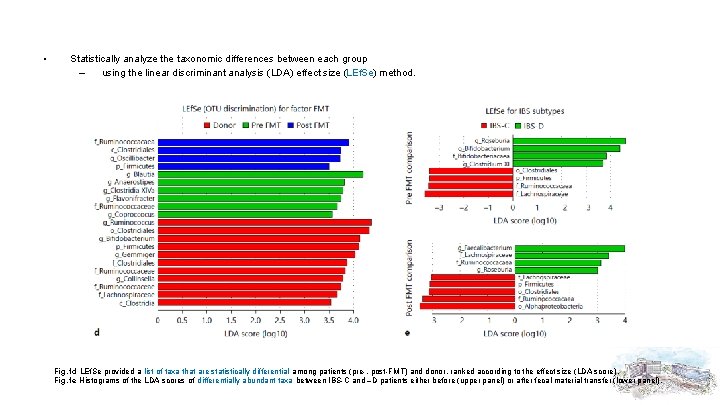
• Statistically analyze the taxonomic differences between each group – using the linear discriminant analysis (LDA) effect size (LEf. Se) method. Fig. 1 d LEf. Se provided a list of taxa that are statistically differential among patients (pre-, post-FMT) and donor, ranked according to the effect size (LDA score). Fig. 1 e Histograms of the LDA scores of differentially abundant taxa between IBS-C and –D patients either before (upper panel) or after fecal material transfer (lower panel).

Beta-diversity • To further characterize taxonomic compositional differences between the donor and patients before and after FMT • Using t-Distributed Stochastic Neighbor Embedding (t. SNE) – • a nonlinear, nonparametric dimensionalityreduction algorithm recently introduced in microbiota data analysis to reveal data-inherent cluster structures Using generalized Uni. Frac distances (among microbiota samples)
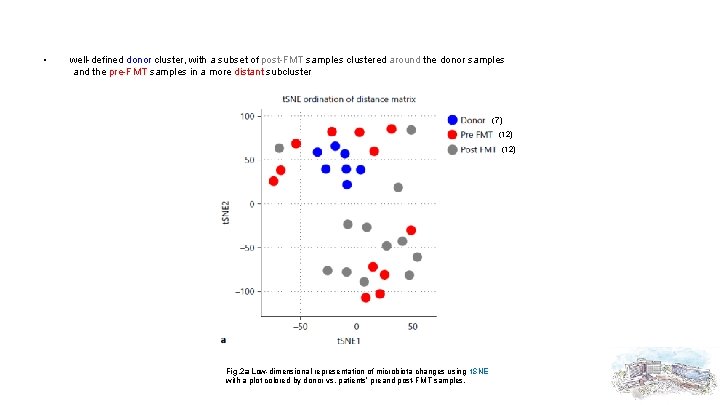
• well-defined donor cluster, with a subset of post-FMT samples clustered around the donor samples and the pre-FMT samples in a more distant subcluster (7) (12) Fig. 2 a Low-dimensional representation of microbiota changes using t. SNE with a plot colored by donor vs. patients’ preand post-FMT samples.
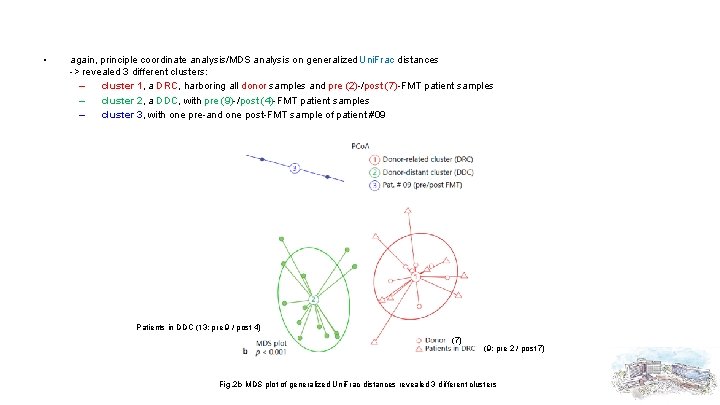
• again, principle coordinate analysis/MDS analysis on generalized Uni. Frac distances -> revealed 3 different clusters: – cluster 1, a DRC, harboring all donor samples and pre (2)-/post (7)-FMT patient samples – cluster 2, a DDC, with pre (9)-/post (4)-FMT patient samples – cluster 3, with one pre-and one post-FMT sample of patient #09 Patients in DDC (13; pre 9 / post 4) (7) (9; pre 2 / post 7) Fig. 2 b MDS plot of generalized Uni. Frac distances revealed 3 different clusters
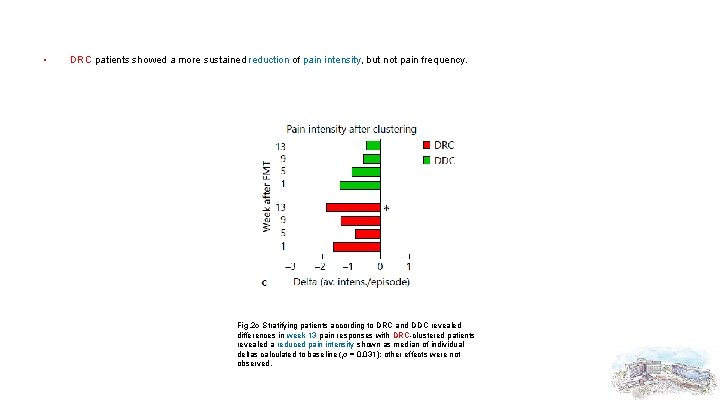
• DRC patients showed a more sustained reduction of pain intensity, but not pain frequency. Fig. 2 c Stratifying patients according to DRC and DDC revealed differences in week 13 pain responses with DRC-clustered patients revealed a reduced pain intensity shown as median of individual deltas calculated to baseline (p = 0. 031); other effects were not observed.
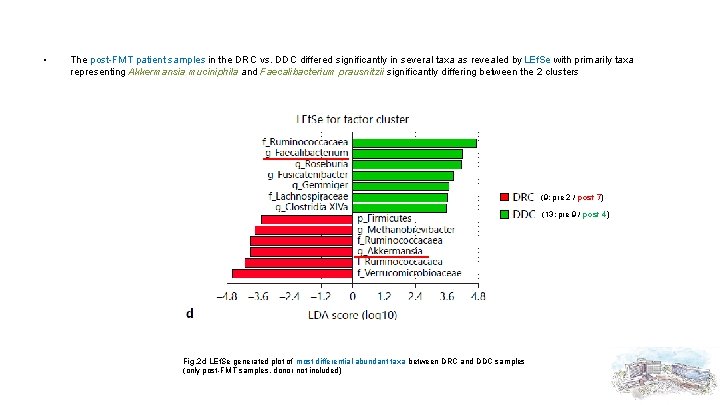
• The post-FMT patient samples in the DRC vs. DDC differed significantly in several taxa as revealed by LEf. Se with primarily taxa representing Akkermansia muciniphila and Faecalibacterium prausnitzii significantly differing between the 2 clusters (9; pre 2 / post 7) (13; pre 9 / post 4) Fig. 2 d LEf. Se generated plot of most differential abundant taxa between DRC and DDC samples (only post-FMT samples, donor not included)
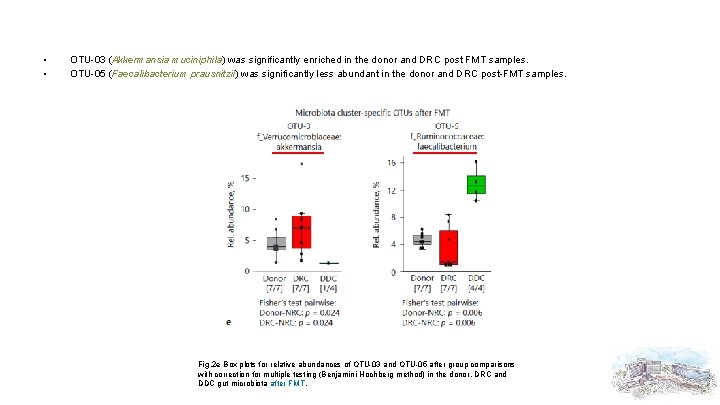
• • OTU-03 (Akkermansia muciniphila) was significantly enriched in the donor and DRC post FMT samples. OTU-05 (Faecalibacterium prausnitzii) was significantly less abundant in the donor and DRC post-FMT samples. Fig. 2 e Box plots for relative abundances of OTU-03 and OTU-05 after group comparisons with correction for multiple testing (Benjamini-Hochberg method) in the donor, DRC and DDC gut microbiota after FMT.
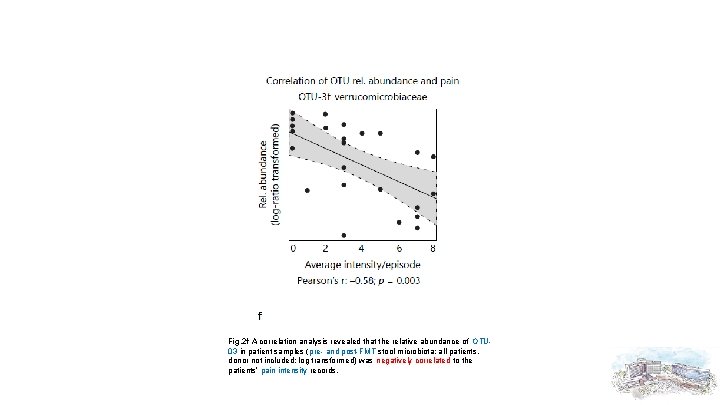
Fig. 2 f A correlation analysis revealed that the relative abundance of OTU 03 in patient samples (pre- and post-FMT stool microbiota; all patients, donor not included; log transformed) was negatively correlated to the patients’ pain intensity records.

Discussion • We found that FMT alleviates abdominal pain in our cohort of IBS patients • A major finding was the observation that patient fecal microbiota profiles clustered closely (DRC) or distantly (DDC) to the donor after FMT – using 2 independent ordination algorithms (t. SNE and MDS). – DRC patients showed a more sustained reduction of abdominal pain

• In line with the concept of a specific donor microbiota factor determining the success of FMT in gastrointestinal disorders – We found mainly Akkermansia and members of the family Ruminococcaceae overrepresented in the donor and DRC samples – whereas Faecalibacterium prausnitzi was not found in this cluster • Therefore, we argue that the taxonomic composition of the donor microbiota is a key factor in the treatment response.

Akkermansia muciniphila • • • a Gram-negative commensal bacterium living in the mucus layer of the intestinal epithelium prevalent in healthy, lean subjects is associated with physical fitness high relative abundances of A. muciniphila inversely correlated with abdominal pain • • • less abundant in IBD significant reduction of A. muciniphila in children with IBS significantly reduced in feces from rats in a model of post-inflammatory IBS • can also promote colitis in mice lacking IL-10 – pointing to a complex, context-dependent action of the bacterium
Pain-alleviating mechanism by A. muciniphila • • One hypothesis; – 2 major short chain fatty acids (SCFA), propionate and acetate, both end products of mucus degradation by Akkermansia, – may modulate visceral nociception – as butyrate stimulation of the SCFA receptor GPR 43 (with equal affinities for acetate, propionate, and butyrate) – decrease visceral pain sensitivity Alternatively; – A. muciniphila can counteract gut mucosal barrier dysfunction in mice – by increasing the intestinal production of endocannabinoids and antimicrobial peptides – or by regulating the endogenous production of gut neuropeptides like glucagon-like peptide 1 and 2 (which were found to be downregulated in IBS)

Conclusion • • We observed a symptom-specific effect of FMT in our cohort of IBS patients that were refractory to conventional treatments. It also highlights the importance of the donor microbiota for treatment success – However, a strategy to select appropriate donors and maximize donor-host microbiome compatibility for a beneficial treatment success still needs to be developed.
Thank you for attention !
- Slides: 50