Jordan Food Drug Administration A Presentation for The





















- Slides: 23

Jordan Food & Drug Administration A Presentation for The Arab Quality & Food Safety Conference June 15 -16, 2006 1

Background As of April, 2003, the Jordan Food and Drug Administration (JFDA) was created by law n The new independent institution (JFDA) took over the following functions n Drug regulatory and quality control functions n Food safety and hygiene n 2

Background n To achieve its function, the following took place the following departments were separated from MOH and became under JFDA jurisdictions n n Drug Directorate (DD) Food Directorate (FD) Quality Control Laboratory (QCL) Food Laboratories including: n n n Amman Aqaba Irbid 3

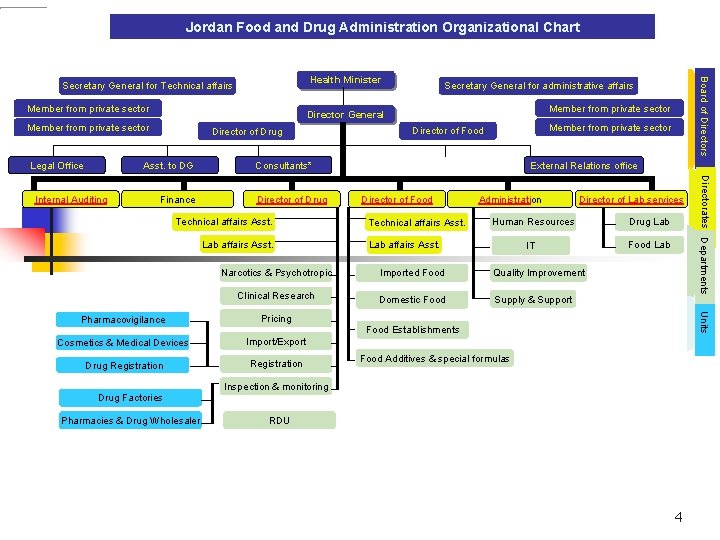
Jordan Food and Drug Administration Organizational Chart Member from private sector Legal Office Director of Drug Asst. to DG Member from private sector Finance Member from private sector Director of Food External Relations office Consultants* Director of Drug Technical affairs Asst. Lab affairs Asst. Director of Food Technical affairs Asst. Administration Director of Lab services Human Resources Drug Lab IT Food Lab affairs Asst. Narcotics & Psychotropic Imported Food Quality Improvement Clinical Research Domestic Food Supply & Support Pricing Cosmetics & Medical Devices Import/Export Drug Registration Units Pharmacovigilance Food Establishments Food Additives & special formulas Inspection & monitoring Drug Factories Pharmacies & Drug Wholesaler Directorates Departments Internal Auditing Secretary General for administrative affairs Director General Member from private sector Board of Directors Health Minister Secretary General for Technical affairs RDU 4

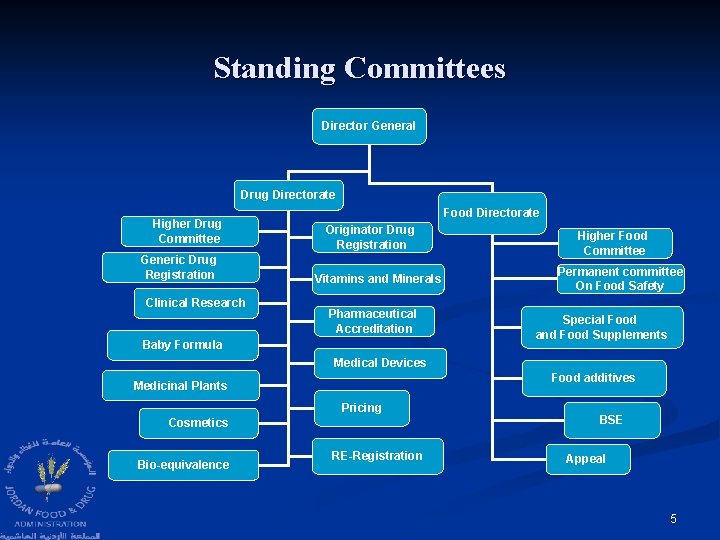
Standing Committees Director General Drug Directorate Higher Drug Committee Generic Drug Registration Clinical Research Food Directorate Originator Drug Registration Vitamins and Minerals Pharmaceutical Accreditation Baby Formula Higher Food Committee Permanent committee On Food Safety Special Food and Food Supplements Medical Devices Food additives Medicinal Plants Pricing Cosmetics Bio-equivalence RE-Registration BSE Appeal 5

Strategic Thinking 6

JFDA Vision JFDA is the leading regulatory institution for safety and quality of food and drugs at the national and regional levels, and recognized at the international level. Gains the trust, and confidence of Jordanian citizens and institutions through the exercise of efficient and effective scientifically based decisions that ensure the public health safety and welfare. 7

Mission: who we are JFDA is an independent public sector regulatory institution whose main objectives are to ensure: 1. 2. 3. that foods are safe, wholesome, and sanitary that drugs are safe, and efficacious the safety of all products explicitly stated in the enforced drug and pharmacy law 8

Core Values 1. 2. 3. 4. 5. Loyalty Achieve Perfection with Self pride Culture of Excellence Transparency Confidentiality 9

Strategies Independence of JFDA financially and administratively Improvement of technical capabilities to achieve international recognition JFDA promotion and awareness Achievement of national integration and international cooperation Rationalization of drug use 10

Preparatory and concomitant requirements n n n Create performance based incentives plan Create a quality improvement culture and IT mentality Create a positive working environment along with human resource training program to move JFDA staff towards more n n n Transparency Objectivity Fairness Accountability Self confidence 11











