Joint AcademiaIndustry Presentation Changing World Changing Pathology Drs

























- Slides: 27

Joint Academia/Industry Presentation “Changing World : Changing Pathology” Drs. Chris Zink (Johns Hopkins) and Esther Trueblood (Amgen) Co-Chairs Drs. Joe Mankowski (Johns Hopkins), Vince Meador (Covance), Lance Perryman (Colorado State), Leah Schutt (Genentech), and Lauren Tierney (Glaxo. Smith. Kline) Panelists 1

Agenda • 3: 45 Chris Zink—Introduction • 3: 50 Vince Meador—Mergers and Acquisitions in Industry • 3: 57 Lance Perryman—Veterinary Pathology in Academia and Departmental Mergers • 4: 04 Joe Mankowski—Comparative Pathology in Academic Medical Centers • 4: 11 Lauren Tierney—Technology Innovations, Data Sharing, and Harmonization • 4: 18 Leah Schutt—The Future of Veterinary Pathology • 4: 25 Audience Participation 2

Vincent Meador Mergers and Acquisitions in Industry • DVM, Ph. D from Iowa State University • Veterinary Medical Officer, USDA, National Animal Disease Center • Principal Research Pathologist, Schering-Plough Research • Director Toxicology and Pathology, Lilly Research Laboratories • Collaborating Professor, Iowa State University • Executive Director, Amgen Inc • Visiting Professor, National Chengdu Center for Safety Evaluation of Traditional Chinese Medicine, China • VP and CSO, Covance Inc 3

Mergers and Acquisitions • Mergers - Example • Acquisitions – Example of Acquiring portion of Toxicology Staff and Laboratory Laboratories

Affects on Pathology • Positions – Decreased number of positions in Pharma – Change in living location • Focus – Pharma decreasing GLP study work • Contract to CROs • Many Pharma retain Discovery and Lead Optimization – Shifts balance of GLP experience and expertise to CRO’s

Lance Perryman Veterinary Pathology in Academia and Departmental Mergers • DVM, Ph. D from Washington State University • Professor, Department of Veterinary Microbiology and Pathology, Washington State University • Associate Dean for Research and Graduate Education, and Director of the Animal Health Research Center, College of Veterinary Medicine, Washington State University • Professor and Head, Department of Microbiology, Pathology, and Parasitology, College of Veterinary Medicine, North Carolina State University • Dean, College of Veterinary Medicine and Biomedical Sciences, Colorado State University 6

Veterinary Pathology in Academia and Departmental Mergers Lance Perryman College of Veterinary Medicine & Biomedical Sciences Colorado State University

Trends in Academia § Reduced state support constrains college and departmental budgets § Response - Merge Pathology with other disciplines and form large departments § CSU combined seven departments into four in 2001 § Non-pathologists (and non-veterinarians) often chair these large departments § Pathologists may lose identity and visibility in large multidisciplinary departments

Microbiology, Immunology and Pathology (MIP) at CSU – a merged department § Formed in 2001 by merging the former Department of Pathology with the former Department of Microbiology

Microbiology, Immunology and Pathology (MIP) at CSU – a merged department § Mission § § Participate heavily in the DVM curriculum Deliver an undergraduate degree in Microbiology Engage in graduate education (MS and Ph. D degrees) Conduct residency training (anatomical and clinical pathology, microbiology) § Staff most of the CSU Diagnostic Laboratory for the State of Colorado § Conduct research, primarily infectious diseases

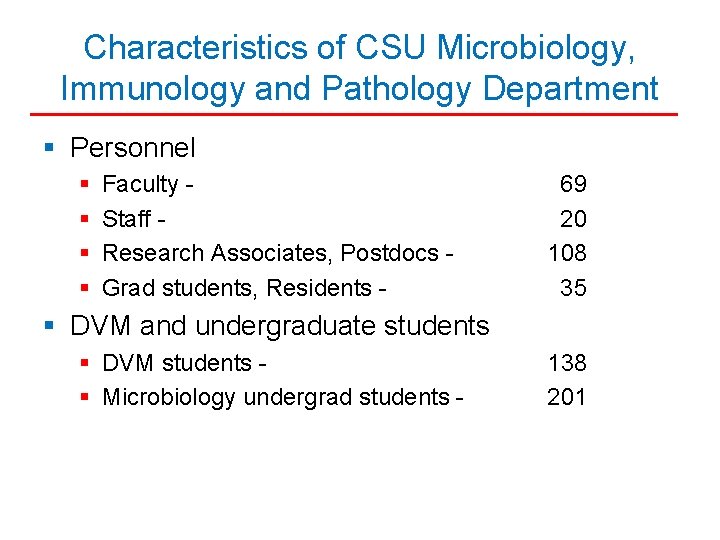
Characteristics of CSU Microbiology, Immunology and Pathology Department § Personnel § § Faculty Staff - Research Associates, Postdocs - Grad students, Residents - 69 20 108 35 § DVM and undergraduate students § DVM students - § Microbiology undergrad students - 138 201

Characteristics of CSU Microbiology, Immunology and Pathology Department § Research portfolio >$30 M annually

MIP Leadership 2001 - Present § Steve Benjamin, DVM, Ph. D, DACVP § Interim Head, 2001 -2003 § Jeff Wilusz, Ph. D § Head, 2003 – 2007; NIH-funded investigator § Ed Hoover, DVM, Ph. D, DACVP, UDP § Head 2008 – 2011; NIH-funded investigator § Gregg Dean, DVM, Ph. D, DACVP § Head, 2011 – present; NIH-funded investigator

Observations on merged departments § Require skilled leadership, often administered through teams § Department Heads lead by example – MIP faculty expect the Head to direct a strong research program funded by NIH § My preference for department head § DVM, Ph. D, DACVP credentials § Supported by grant funds from NIH

Joseph Mankowski Comparative Pathology in Academic Medical Centers • DVM, Cornell University • Ph. D Johns Hopkins University, • Associate Professor, Johns Hopkins University – Department of Molecular & Comparative Pathobiology – Department of Pathology – Department of Neurology • Visiting Scientist, University of Southampton, UK 15

Comparative Pathology In Academic Medical Centers

Opportunities A spectrum of classic Comparative Medicine roles • • Clinical diagnostic support – partnership with LAM Teaching mission – DVM , MD, Ph. D Collaborative research teams: experimental pathology Translational science - cutting edge interdisciplinary teams • Principal Investigator role

Challenges • • Funding for research and training programs NCRR uncertainty Recruiting trainees with a passion for research Recruiting and retaining faculty


Lauren Tierney Technology Innovations, Data Sharing, and Harmonization • DVM from Tufts University • Ph. D from University of New Mexico, Inhalation Toxicology • Residency Johns Hopkins University, NSRA service grant • Director of Pathology, Glaxo. Smith. Kline 20

The Future of Veterinary Pathology Technology Innovations, Data Sharing and Harmonization ACVP/STP Coalition Symposium 2011 Lauren Tierney DVM, Ph. D, DACVP Glaxo. Smith. Kline

The Future of Veterinary Pathology • Technology Innovations: Digital Pathology • Data Sharing: SEND (The Future of Electronic Data Submissions) • Harmonization: INHAND Initiative

Technology Innovations: Digital Pathology § Scanning of glass slide content into digital slide images § New generation digital microscopes with server interface Image. Scope/Scanscope § Challenges: limiting bandwidth, slow refresh rate; acceptance; validation requirement for whole slide imaging in regulated environments § Opportunities: Facilitates/Accelerates interaction at remote sites/emerging R&D centers; allows slide review without travel (remember Icelandic volcano? ); No CITES permit requirement (NHP material) § Digital Pathology Association ((http: //digitalpathologyassociation. org) § “Validation of Digital pathology systems in the regulated nonclinical environment “

Technology Innovations: continued Will it be possible to submit digitally “read” studies to regulatory agencies ? FDA digital pathology workforce § Concerns about the accuracy and reproducibility of digital slide images vs. glass slides § Currently categorizes whole slide imaging devices as Class III (sufficient information not available to insure safety and effectiveness through the application of general and specific controls) § Concern for patient safety if digital slide is read incorrectly (primarily clinical concern but has similar implications in digital peer review of regulated nonclinical studies) § Awaiting Final decision!

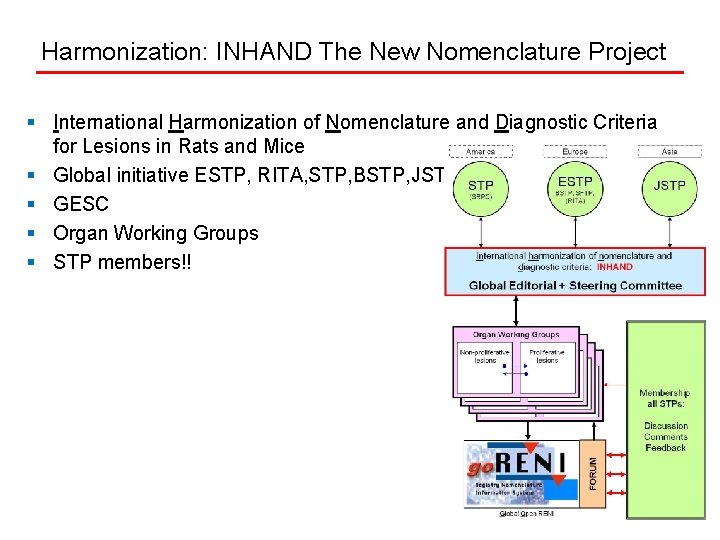
Harmonization: INHAND The New Nomenclature Project § International Harmonization of Nomenclature and Diagnostic Criteria for Lesions in Rats and Mice § Global initiative ESTP, RITA, STP, BSTP, JSTP § GESC § Organ Working Groups § STP members!!

Data Sharing: SEND Standard for Exchange of Nonclinical Data • The Future of Electronic Data Submissions (including nonclinical pathology tabular data) • Proposed regulatory Standard for Exchange of Nonclinical Data sponsored by a data interchange standards consortium (CDISC). • Goal to have standards for nonclinical data exchange, not just with the regulatory agencies but also between laboratories • FDA is recommending, although not yet required • FDA and INHAND Steering Committee ongoing discussions on potential use of INHAND terminology as preferred terminology for SEND • Potential benefits – Streamlined process from report to submission, quicker regulatory review, ability to incorporate external data in-house study data warehouse, more efficient way to share data with the various consortium, • Logistics of translating existing data streams into the SEND format

Leah Schutt The Future of Veterinary Pathology • DVM, DVSc from University of Guelph, ON, Canada • ACVP/STP Coalition Fellowship, – Industry Sponsor Genentech • Scientist, Genentech Inc 27