JEOPARDY Third Nine Weeks 9 weeks review El




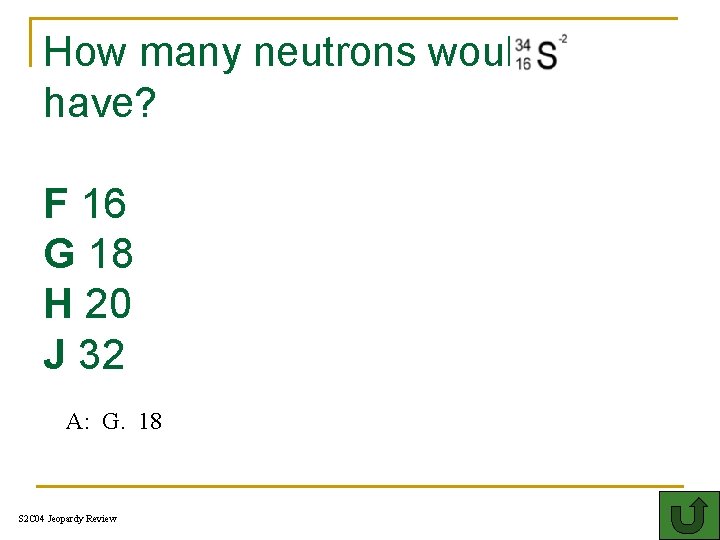





- Slides: 27

JEOPARDY Third Nine Weeks 9 weeks review

El Dorado High School AZTECS 100 100 100 200 200 200 300 300 300 400 400 400 500 500 500

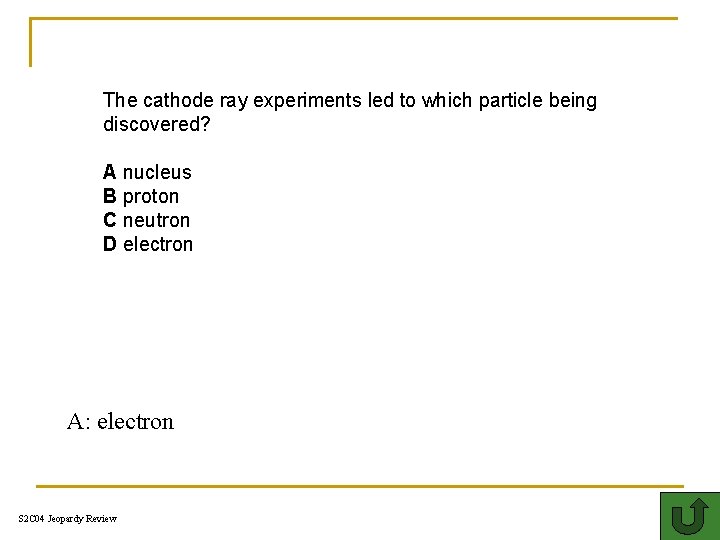
The cathode ray experiments led to which particle being discovered? A nucleus B proton C neutron D electron A: electron S 2 C 04 Jeopardy Review

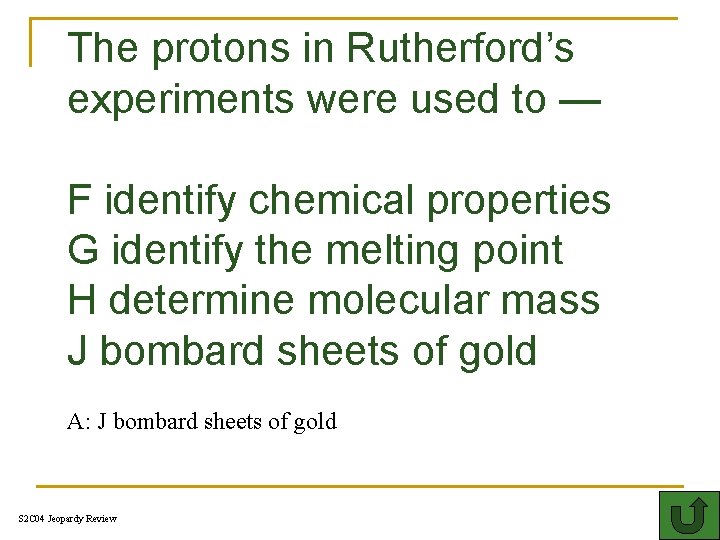
The protons in Rutherford’s experiments were used to — F identify chemical properties G identify the melting point H determine molecular mass J bombard sheets of gold A: J bombard sheets of gold S 2 C 04 Jeopardy Review

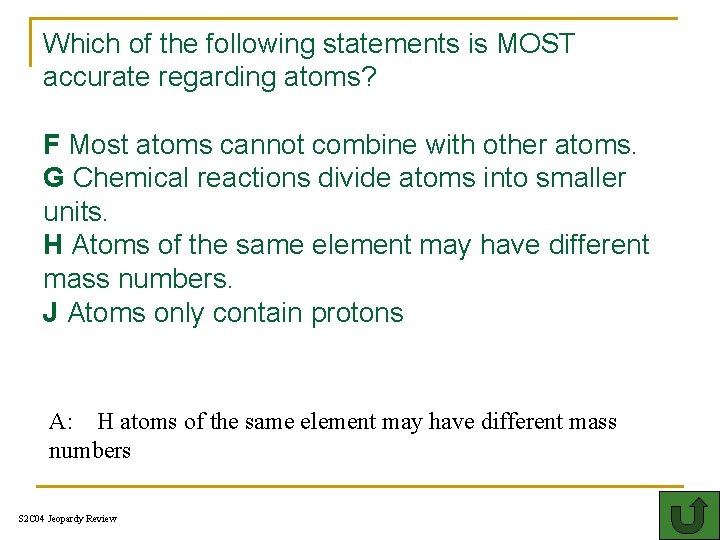
Which of the following statements is MOST accurate regarding atoms? F Most atoms cannot combine with other atoms. G Chemical reactions divide atoms into smaller units. H Atoms of the same element may have different mass numbers. J Atoms only contain protons A: H atoms of the same element may have different mass numbers S 2 C 04 Jeopardy Review

Which type of solution has achieved equilibrium between solute and solvent if the solute is still visible after mixing? F an unsaturated solution G a saturated solution H a supersaturated solution J a dilute solution A: G. A saturated solutin S 2 C 04 Jeopardy Review

A solution that contains the maximum amount of solute that is soluble at a given temperature is said to be — F unsaturated G saturated H supersaturated J diluted 500 A: G. saturated S 2 C 04 Jeopardy Review

Which substance would be least likely to dissolve in a polar solvent? F nonpolar molecule G acids H polar molecule J ionic compound 00 A: S 2 C 04 Jeopardy Review F. nonpolar molecule

Using the periodic table, predict which elements will have similar chemical properties or reactivity. F cadmium, calcium, and carbon G magnesium, strontium, and barium H rubidium, yttrium, and zirconium J nitrogen, sulfur, and bromine 200 A: G S 2 C 04 Jeopardy Review

How many m. L of stock NH 3 is needed to make 100. 0 m. L of 1. 00 M NH 3? Your stock solution is 14. 8 M. (Answer in significant figures with m. L for unit. ) 300 A: 6. 76 m. L S 2 C 04 Jeopardy Review

The number of valence electrons in Group 2 elements is — F 0 G 1 H 2 J 3 I A: H. 2 400 S 2 C 04 Jeopardy Review

What happens to the number of electrons in an atom as the atomic number on the Periodic Table increases? A THIS increases B decreases C remains the same D depends on the atomo 500 A: A. increases S 2 C 04 Jeopardy Review

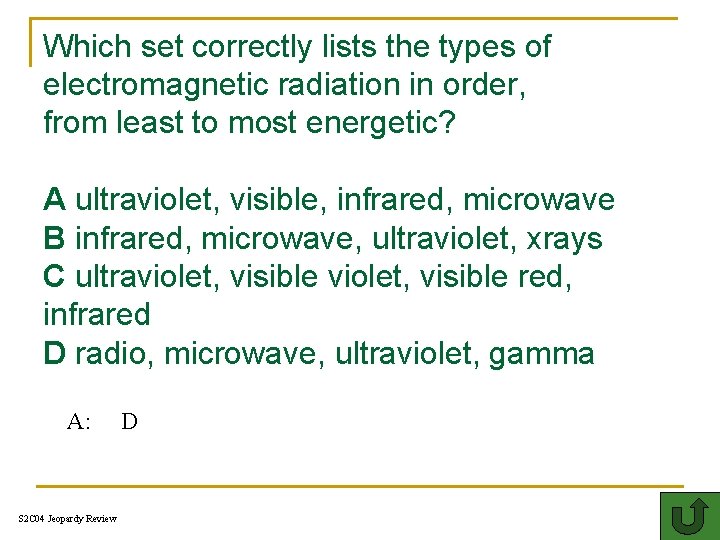
Which set correctly lists the types of electromagnetic radiation in order, from least to most energetic? A ultraviolet, visible, infrared, microwave TH B infrared, microwave, ultraviolet, xrays C ultraviolet, visible red, infrared D radio, microwave, ultraviolet, gamma A: S 2 C 04 Jeopardy Review D
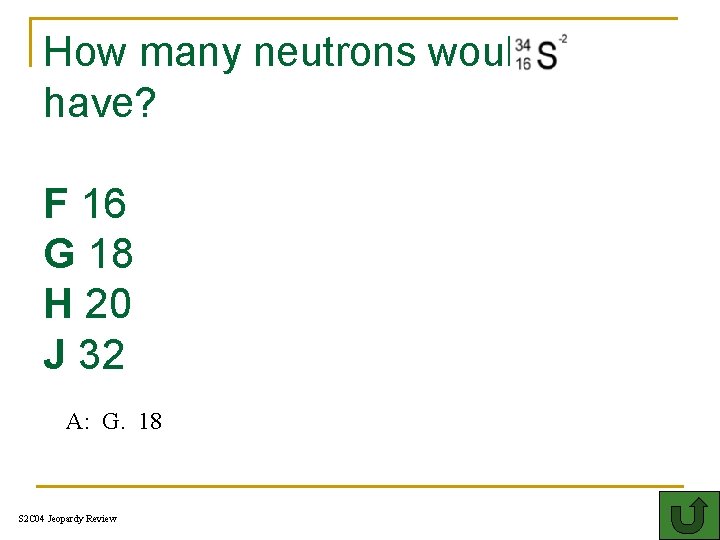
How many neutrons would have? F 16 G 18 H 20 J 32 A: G. 18 S 2 C 04 Jeopardy Review

Which of the following is TRUE about Nickel-59? A 28 neutrons B 28 protons C 31 electrons D 59 neutrons A: S 2 C 04 Jeopardy Review B. ) 28 protons

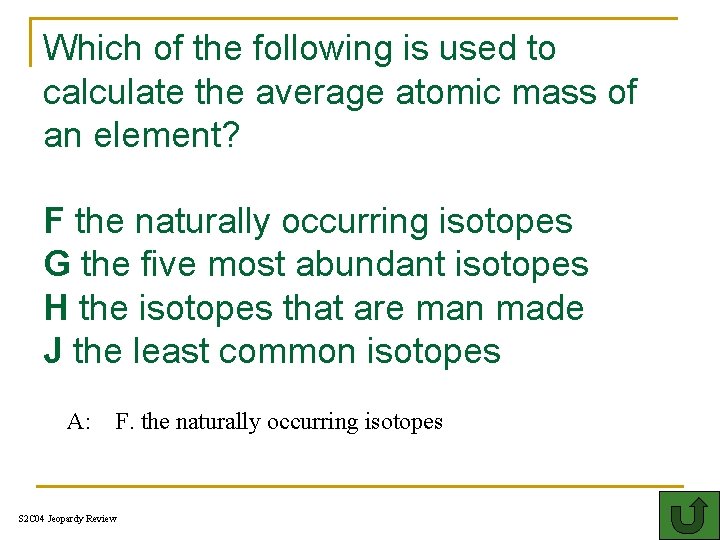
Which of the following is used to calculate the average atomic mass of an element? FH the naturally occurring isotopes G the five most abundant isotopes H the isotopes that are man made J the least common isotopes A: F. the naturally occurring isotopes S 2 C 04 Jeopardy Review

Which of the following atoms would have the largest ionic radius? A lithium B sodium CITY. C potassium D cesium 00 A: D. Cesium S 2 C 04 Jeopardy Review

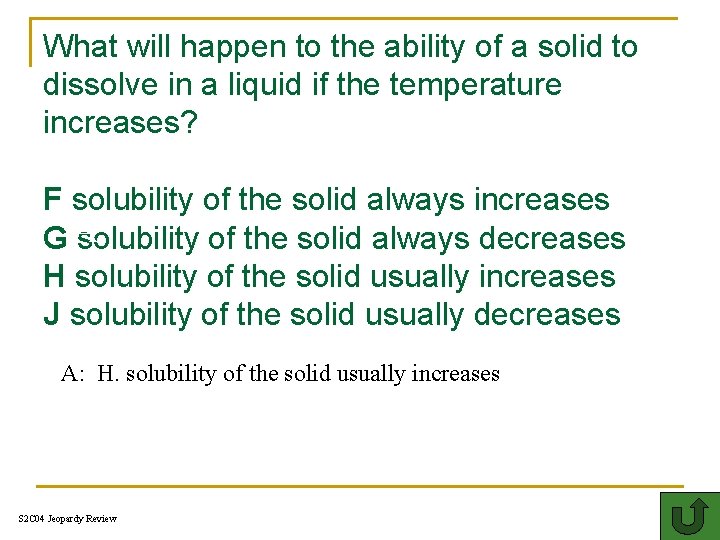
What will happen to the ability of a solid to dissolve in a liquid if the temperature increases? F solubility of the solid always increases GA solubility of the solid always decreases H solubility of the solid usually increases J solubility of the solid usually decreases A: H. solubility of the solid usually increases S 2 C 04 Jeopardy Review

A solution in which more solute is dissolved than is typically soluble at a given temperature is — AA unsaturated B saturated C supersaturated D diluted A. C. supersaturated S 2 C 04 Jeopardy Review

Which type of solution has achieved equilibrium between solute and solvent if the solute is still visible after mixing? F an unsaturated solution G a saturated solution H a supersaturated solution J a dilute solution A: S 2 C 04 Jeopardy Review G. A saturated solution

A sample of 0. 0255 mol potassium hydroxide, KOH, was dissolved in water to yield 10. 0 m. L of solution. What is the molarity of the solution? A Th 2. 55 M B 2. 55 x 103 M C 392 M D. 392 M A: S 2 C 04 Jeopardy Review 2. 55 M

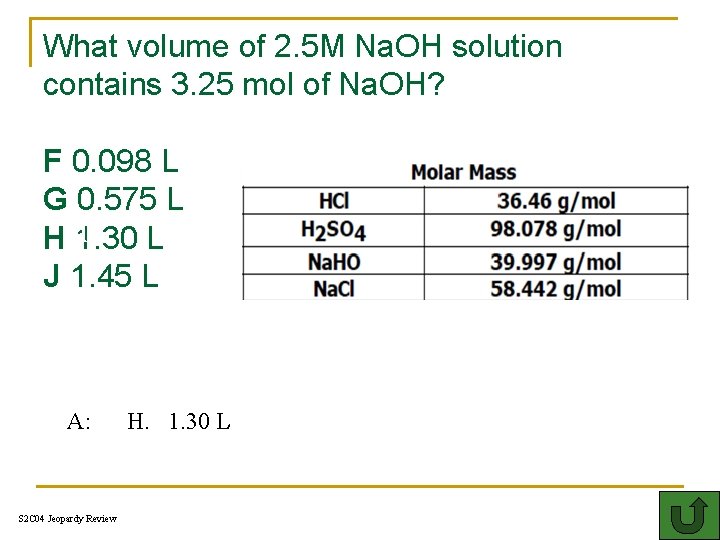
What volume of 2. 5 M Na. OH solution contains 3. 25 mol of Na. OH? F 0. 098 L G 0. 575 L HT 1. 30 L J 1. 45 L A: S 2 C 04 Jeopardy Review H. 1. 30 L

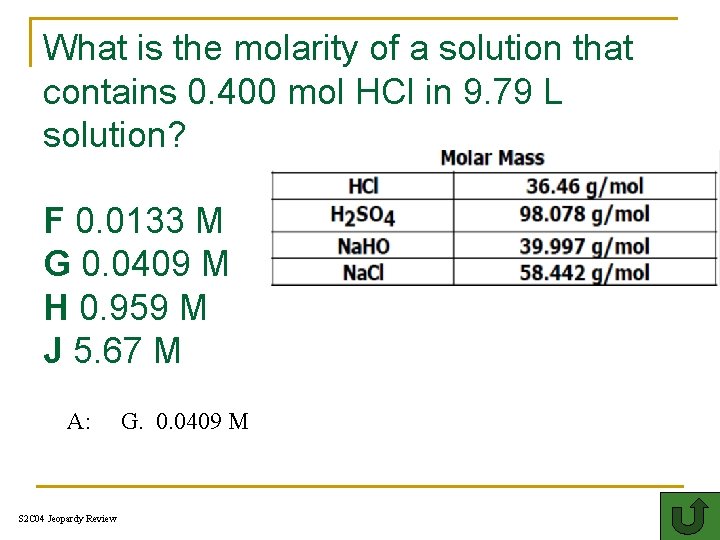
What is the molarity of a solution that contains 0. 400 mol HCl in 9. 79 L solution? F 0. 0133 M G 0. 0409 M H 0. 959 M J 5. 67 M A: S 2 C 04 Jeopardy Review G. 0. 0409 M

2 Students are using heat, chemicals, and glassware in a laboratory experiment. Which of these is the BEST method of eye protection? T F safety goggles G contact lenses H prescription eye glasses J no eye protection needed 00 A: S 2 C 04 Jeopardy Review F. Safety goggles

An isotope of mercury has 80 protons and 120 neutrons. What is the mass number of this isotope? T A 80 B 120 C 200 D A: 201 C. 200 S 2 C 04 Jeopardy Review

Students complete a laboratory investigation. Who is responsible for returning the materials to their proper places? AT students who did the investigation B students entering the laboratory C building services personnel D the chemistry teacher A: S 2 C 04 Jeopardy Review A. students who did the investigation

Why is an atom considered electrically neutral? F neutrons equal the number of protons G proton forces pull on the neutrons HC electrons equal the number of protons J electrons equal the number of neutrons 0 A: S 2 C 04 Jeopardy Review H. Electrons equal the number of protons