JEOPARDY Third Nine Weeks 6 weeks review El













- Slides: 27

JEOPARDY Third Nine Weeks 6 weeks review

El Dorado High School AZTECS 100 100 100 200 200 200 300 300 300 400 400 400 500 500 500

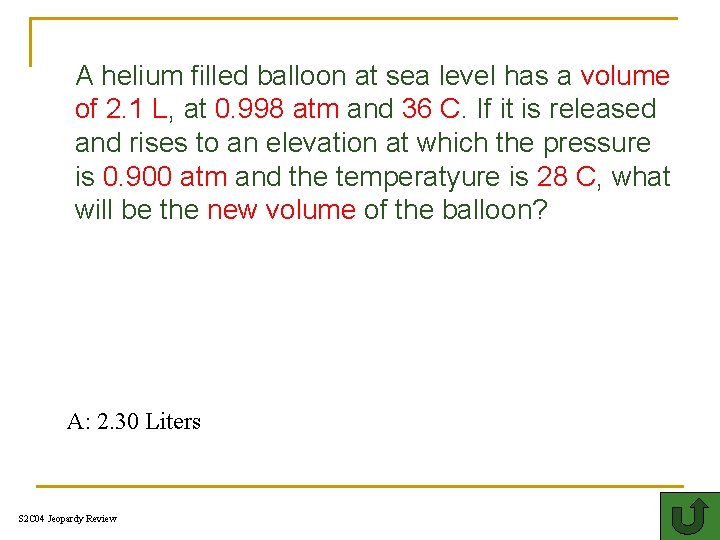
A helium filled balloon at sea level has a volume of 2. 1 L, at 0. 998 atm and 36 C. If it is released and rises to an elevation at which the pressure is 0. 900 atm and the temperatyure is 28 C, what will be the new volume of the balloon? A: 2. 30 Liters S 2 C 04 Jeopardy Review

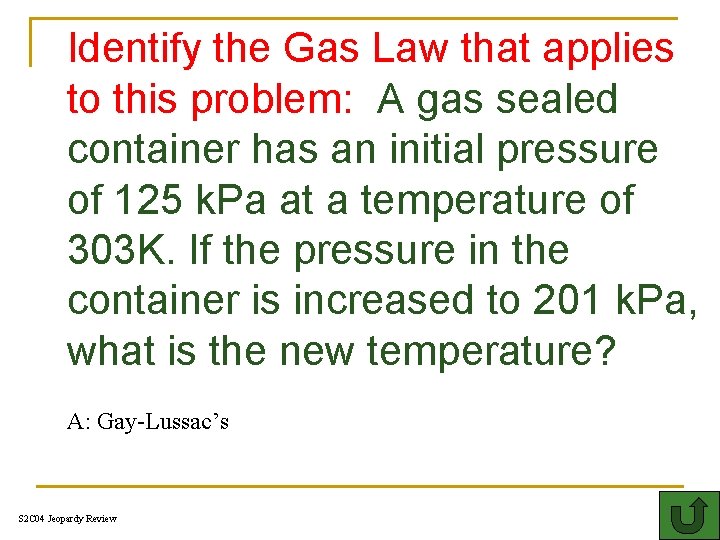
Identify the Gas Law that applies to this problem: A gas sealed container has an initial pressure of 125 k. Pa at a temperature of 303 K. If the pressure in the container is increased to 201 k. Pa, what is the new temperature? A: Gay-Lussac’s S 2 C 04 Jeopardy Review

Air trapped in a cylinder fitted with a piston occupies an initial volume of 145. 7 m. L at an initial pressure of 1. 08 atm pressure. What is the new volume of air when the pressure is increased to 1. 43 atm by applying force to the piston? A: 110. 04 m. L S 2 C 04 Jeopardy Review

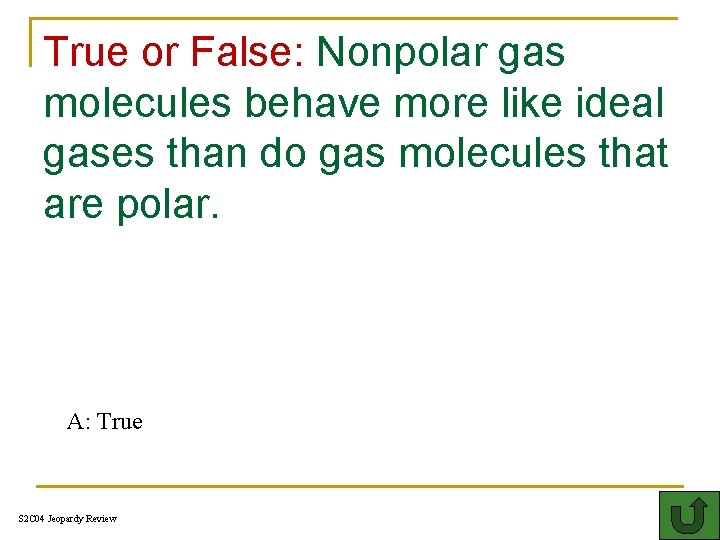
True or False: Nonpolar gas molecules behave more like ideal gases than do gas molecules that are polar. 400 A: True S 2 C 04 Jeopardy Review

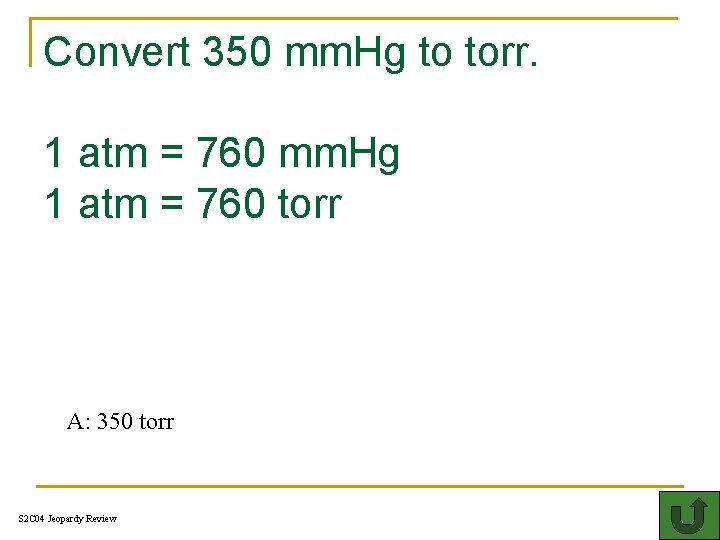
Convert 350 mm. Hg to torr. 1 atm = 760 mm. Hg 1 atm = 760 torr 500 A: 350 torr S 2 C 04 Jeopardy Review

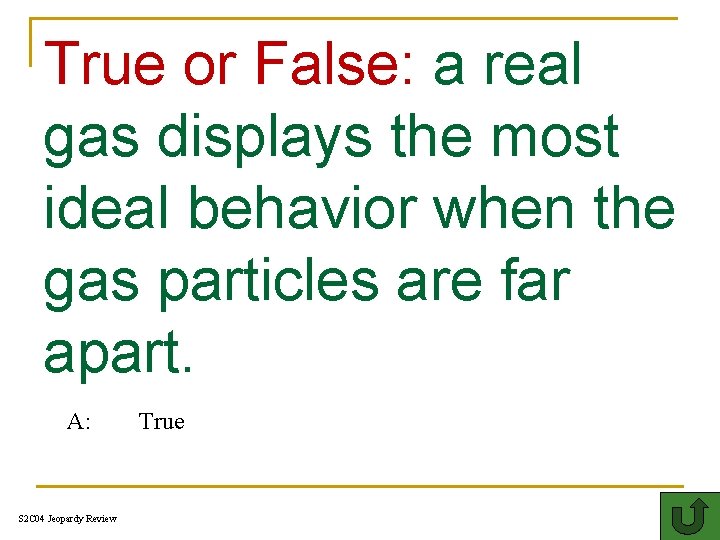
True or False: a real gas displays the most ideal behavior when the gas particles are far apart. 100 A: S 2 C 04 Jeopardy Review True

Which of the following describes pressure: A. ) the force per unit of volume B. ) the force per unit of temperature C. ) the force per unit of surface area A: C D. ) the force per unit of depth S 2 C 04 Jeopardy Review

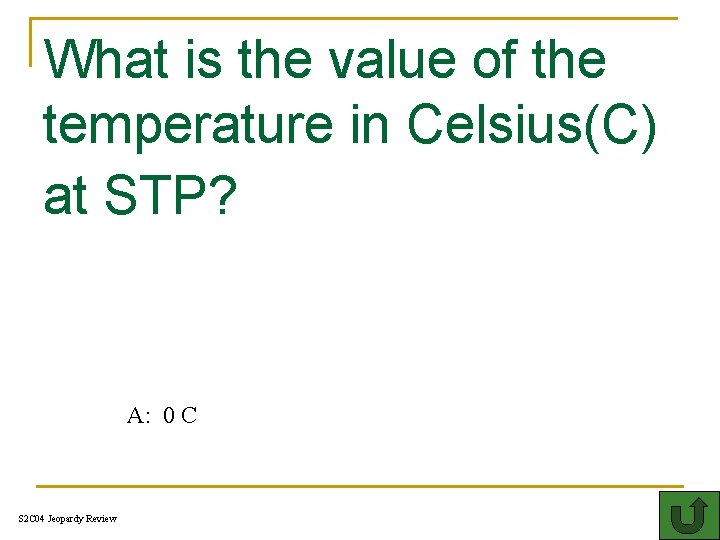
What is the value of the temperature in Celsius(C) at STP? 300 A: 0 C S 2 C 04 Jeopardy Review

Which of the following gas laws is calculated with the pressure and volume variables at a constant temperature? A. ) Charles’s law B. ) Boyle’s law C. ) Gay-Lussac’s law D. ) Dalton’s law A: Boyle’s law S 2 C 04 Jeopardy Review

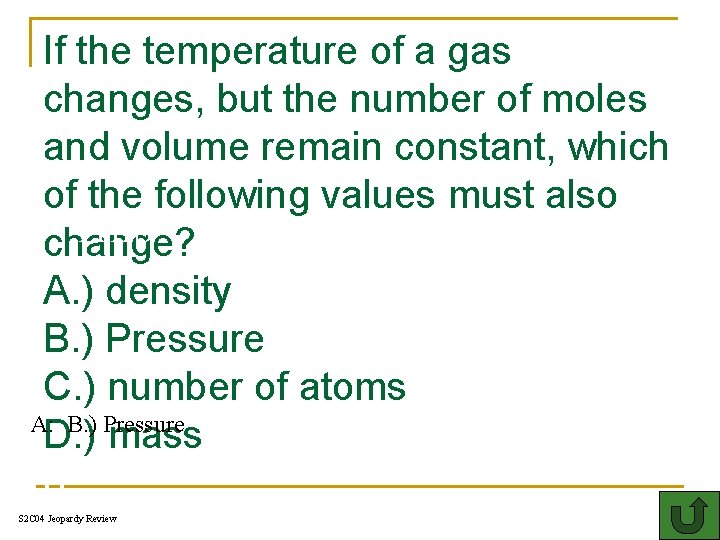
If the temperature of a gas changes, but the number of moles and volume remain constant, which of the following values must also THIS change? A. ) density B. ) Pressure C. ) number of atoms A: B. ) Pressure D. ) mass o S 2 C 04 Jeopardy Review

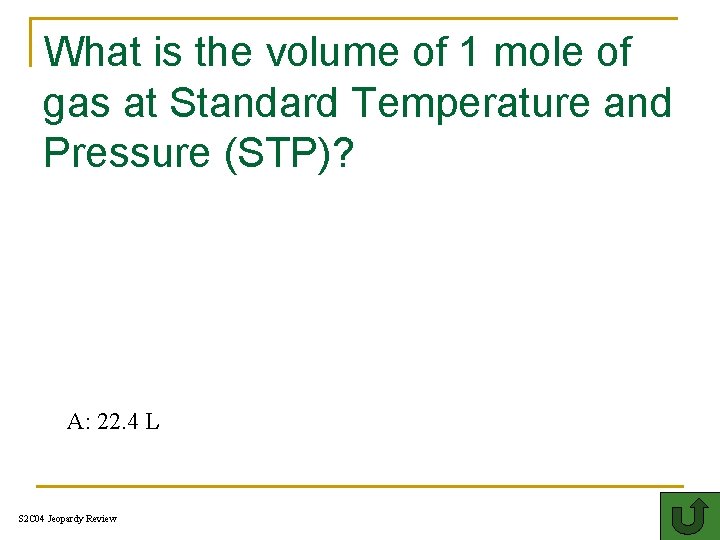
What is the volume of 1 mole of gas at Standard Temperature and Pressure (STP)? TH A: 22. 4 L S 2 C 04 Jeopardy Review

The volume of a gas is 50. 0 m. L at 20. 0 K. What will be the new temperature if the gas is compressed to 10. 0 m. L under constant pressure? A: 4. 00 K S 2 C 04 Jeopardy Review

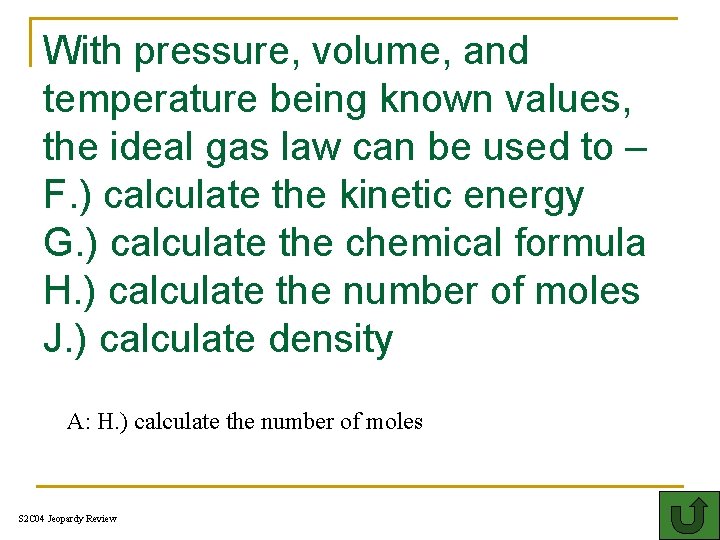
With pressure, volume, and temperature being known values, the ideal gas law can be used to – F. ) calculate the kinetic energy G. ) calculate the chemical formula H. ) calculate the number of moles J. ) calculate density A: H. ) calculate the number of moles S 2 C 04 Jeopardy Review

What is the value of pressure (in atm) at STP? H A: 1 atm S 2 C 04 Jeopardy Review

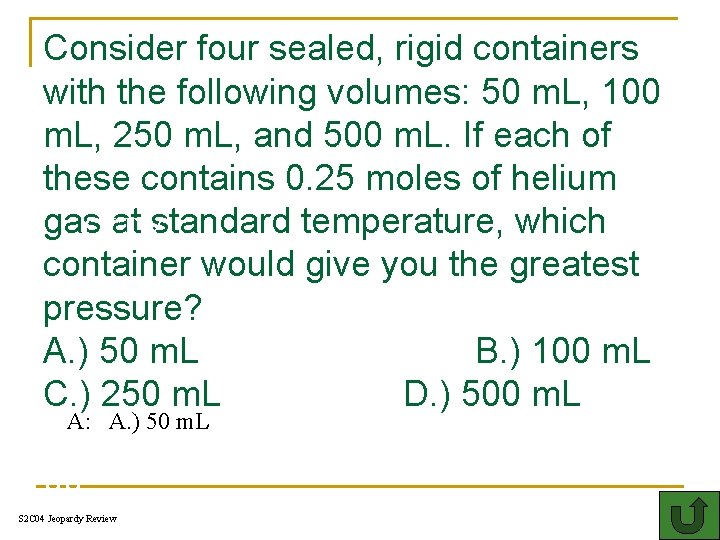
Consider four sealed, rigid containers with the following volumes: 50 m. L, 100 m. L, 250 m. L, and 500 m. L. If each of these contains 0. 25 moles of helium gas at standard temperature, which CITY. container would give you the greatest pressure? A. ) 50 m. L B. ) 100 m. L C. ) 250 m. L D. ) 500 m. L A: A. ) 50 m. L 00 S 2 C 04 Jeopardy Review

The ideal gas law assumes that there are no forces of attraction or repulsion between gas particles. Which of the following best explains why water vapor deviates from the behavior expected of A an ideal gas? A: A. ) it is a polar gas B. ) it is a diatomic gas C. )it is an ionized gas D. ) it is an unstable gas S 2 C 04 Jeopardy Review

The volume of gas in a balloon is 1. 90 L at 21. 0 C. The balloon is heated, causing it to expand to a new volume of 5. 70 L. What is the A new temperature (in Celsius)of the gas inside the new balloon? 200 A. 609 C S 2 C 04 Jeopardy Review

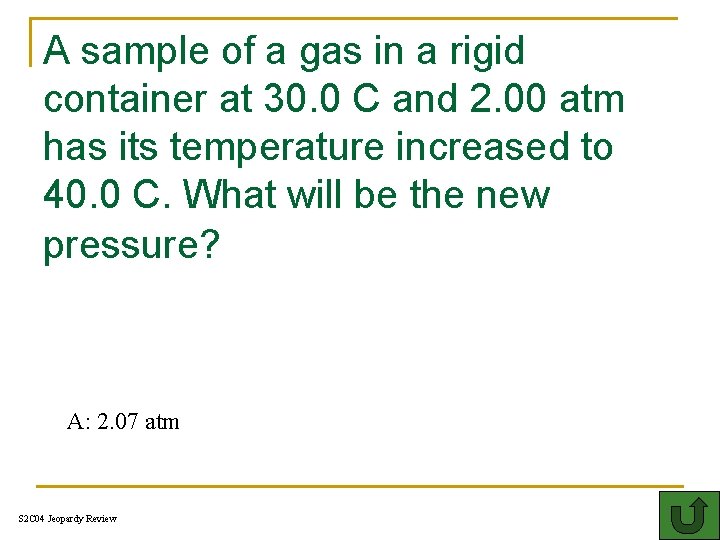
A sample of a gas in a rigid container at 30. 0 C and 2. 00 atm has its temperature increased to 40. 0 C. What will be the new pressure? 300 A: 2. 07 atm S 2 C 04 Jeopardy Review

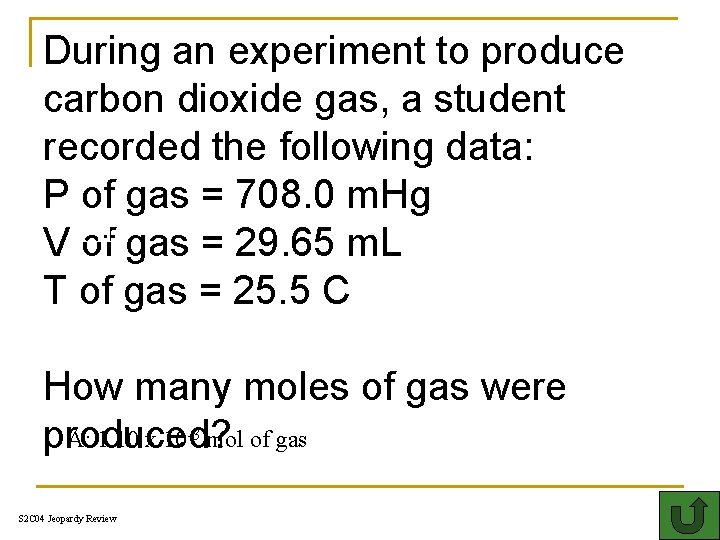
During an experiment to produce carbon dioxide gas, a student recorded the following data: P of gas = 708. 0 m. Hg Th V of gas = 29. 65 m. L T of gas = 25. 5 C How many moles of gas were A: 1. 10 x 10 mol of gas produced? -3 S 2 C 04 Jeopardy Review

What gas law would be used to solve the following problem: A sample of gas is found to have an initial volume of 3. 00 L. The T temperature of the sample is lowered from 353 K to 303 K. What will be the final volume of this gas? 500 A: Charles’s law S 2 C 04 Jeopardy Review

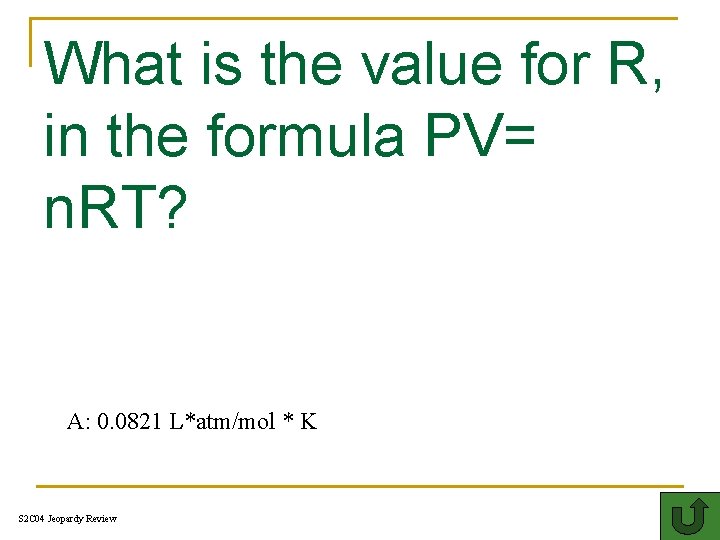
What is the value for R, in the formula PV= n. RT? G 100 A: 0. 0821 L*atm/mol * K S 2 C 04 Jeopardy Review

What units are used when solving for pressure? T 200 A: mm. Hg, atm, torr, k. Pa S 2 C 04 Jeopardy Review

Convert 330 C to Kelvin T A: 603 K S 2 C 04 Jeopardy Review

Convert 405 K to Celsius 400 T A: S 2 C 04 Jeopardy Review 132 C

Manipulate the Ideal Gas Law so that you solve for the number of moles. 500 C A: PV/ RT= n S 2 C 04 Jeopardy Review