Jeopardy Mixed Topics Endothermic Exothermic Reaction Rates Energy















































- Slides: 53

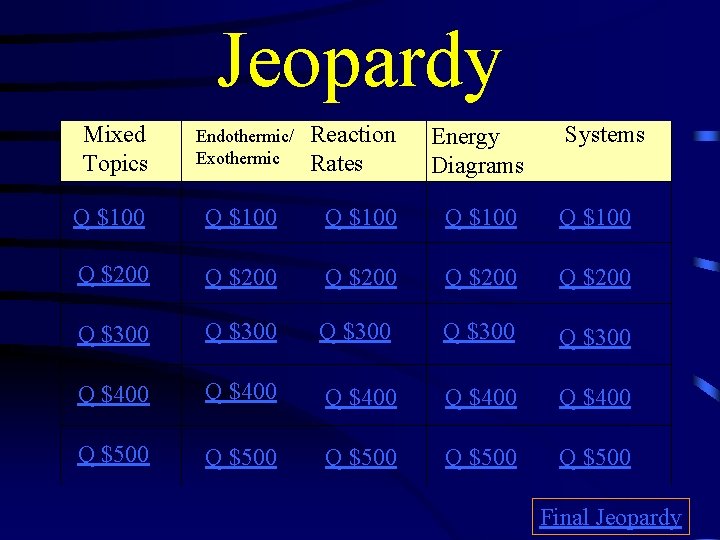
Jeopardy Mixed Topics Endothermic/ Exothermic Reaction Rates Energy Diagrams Systems Q $100 Q $100 Q $200 Q $200 Q $300 Q $300 Q $400 Q $400 Q $500 Q $500 Final Jeopardy

$100 Question from H 1 What is activation energy?

$100 Answer from H 1 Energy required to start reaction

$200 Question from H 1 If the particles of a sample are moving Faster, how has the temperature changed?

$200 Answer from H 1 increased

$300 Question from H 1 If a catalyzed reaction stops and you Add more of the reactants, do you Need to add more of the catalyst? Explain.

$300 Answer from H 1 No. Catalyst isn’t used.

$400 Question from H 1 How does the temperature of a Substance change when a phase Change is occurring?

$400 Answer from H 1 Stays the same

$500 Question from H 1 What three phase changes increase In energy? I want the names!

$500 Answer from H 1 Melting, vaporization, sublimation

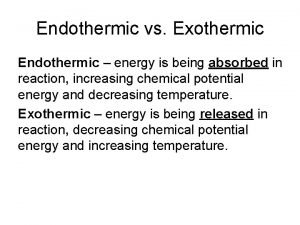
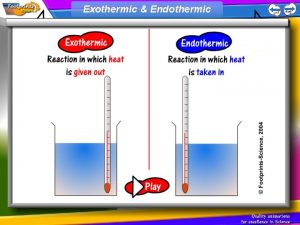
$100 Question from H 2 What happens in endothermic and Exothermic reactions?

$100 Answer from H 2 Endothermic- gains energy Exothermic- loses energy

$200 Question from H 2 How will a flask feel if an exothermic Reaction is happening inside it?

$200 Answer from H 2 hot

$300 Question from H 2 How will a flask feel if an endothermic Reaction is going on inside it?

$300 Answer from H 2 cold

$400 Question from H 2 In an endothermic reaction, do the Products have more or less energy Than the reactants?

$400 Answer from H 2 more

$500 Question from H 2 In an exothermic reaction, do the Products have more or less energy Than the reactants?

$500 Answer from H 2 less

$100 Question from H 3 How does increasing the concentration Affect the reaction rate?

$100 Answer from H 3 increases

$200 Question from H 3 How does increasing particle size Affect the reaction rate?

$200 Answer from H 3 decreases

$300 Question from H 3 Why does increasing temperature Increase the reaction rate?

$300 Answer from H 3 Faster particles, more collisions and More particles have activation energy

$400 Question from H 3 Why does increasing concentration Increase the reaction rate?

$400 Answer from H 3 More particles, more collisions

$500 Question from H 3 How does a catalyst increase the Reaction rate?

$500 Answer from H 3 Decreasing activation energy

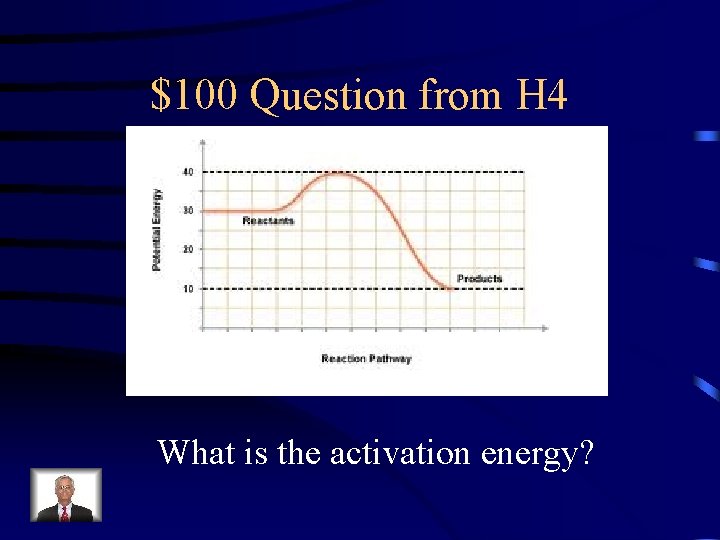
$100 Question from H 4 What is the activation energy?

$100 Answer from H 4 10 k. J

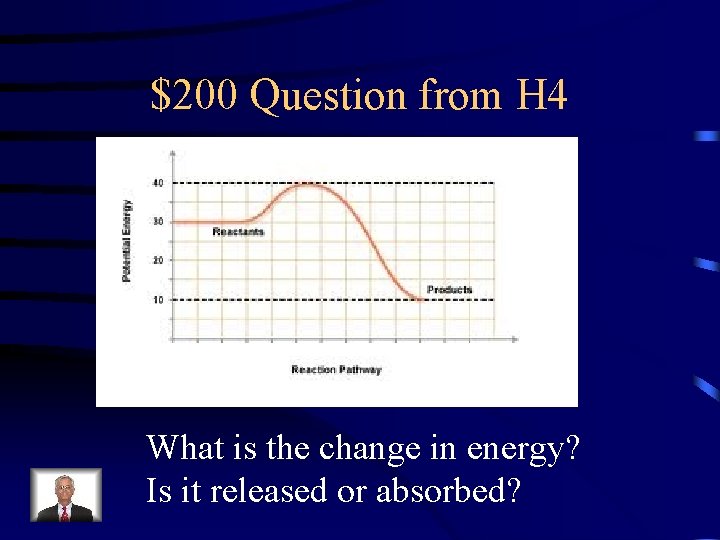
$200 Question from H 4 What is the change in energy? Is it released or absorbed?

$200 Answer from H 4 20 k. J released

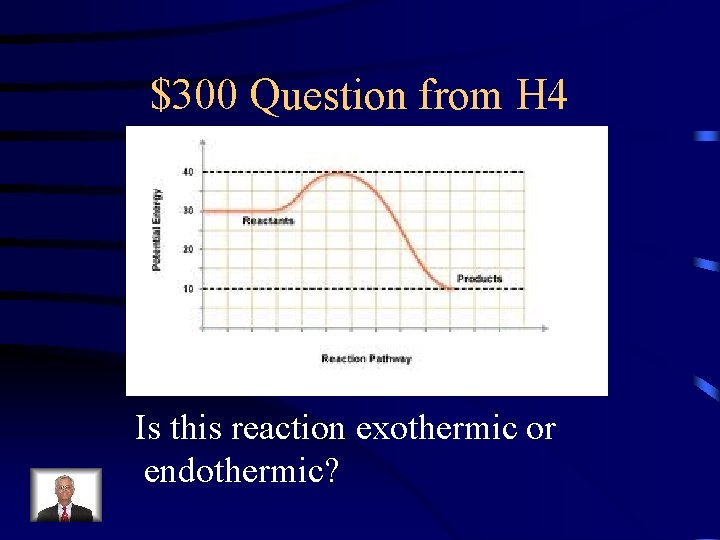
$300 Question from H 4 Is this reaction exothermic or endothermic?

$300 Answer from H 4 exothermic

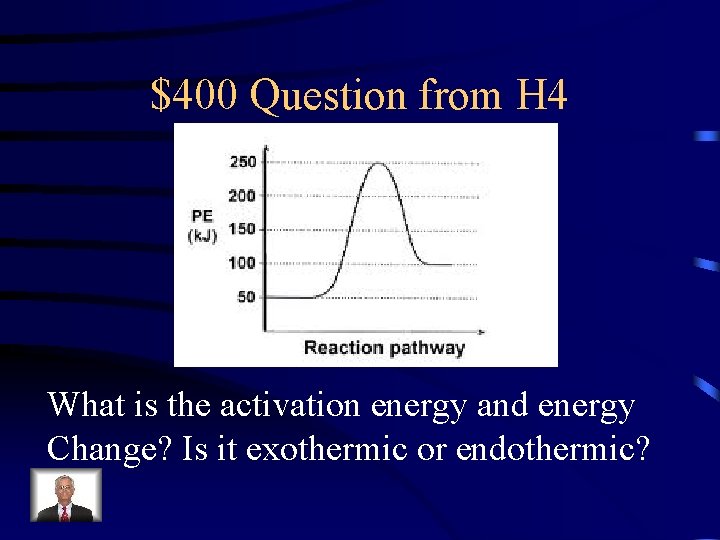
$400 Question from H 4 What is the activation energy and energy Change? Is it exothermic or endothermic?

$400 Answer from H 4 200 k. J, 50 k. J absorbed, endothermic

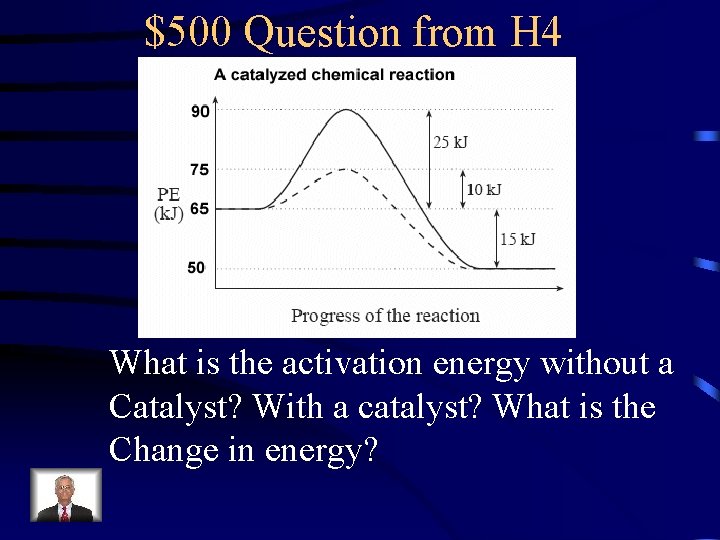
$500 Question from H 4 What is the activation energy without a Catalyst? With a catalyst? What is the Change in energy?

$500 Answer from H 4 25 k. J, 10 k. J, 15 k. J

$100 Question from H 5 What are three types of systems?

$100 Answer from H 5 Open, closed, isolated

$200 Question from H 5 What is a closed system?

$200 Answer from H 5 Energy exchange, not matter

$300 Question from H 5 What is an isolated system?

$300 Answer from H 5 Energy and matter don’t exchange

$400 Question from H 5 What is an open system?

$400 Answer from H 5 Energy and matter exchange

$500 Question from H 5 Give two examples for open, Closed, and isolated systems.

$500 Answer from H 5 Answers Vary

Final Jeopardy Draw an energy diagram where the reactants Have an energy of 25 k. J, the activation energy Is 60 k. J, and the reaction absorbs 30 k. J of Energy. Is the reaction endothermic or exothermic?

Final Jeopardy Answer endothermic
 Endothermic and exothermic reaction experiment grade 11
Endothermic and exothermic reaction experiment grade 11 Which of these phase changes is an endothermic process?
Which of these phase changes is an endothermic process? Endothermic vs exothermic
Endothermic vs exothermic Methane oxygen endothermic or exothermic
Methane oxygen endothermic or exothermic Exothermic v endothermic
Exothermic v endothermic Is vaporization endothermic or exothermic
Is vaporization endothermic or exothermic How to determine exothermic or endothermic
How to determine exothermic or endothermic Endothermic vs exothermic animals
Endothermic vs exothermic animals Endothermic and exothermic worksheet
Endothermic and exothermic worksheet Exothermic and endothermic homework
Exothermic and endothermic homework Cellular respiration endothermic or exothermic
Cellular respiration endothermic or exothermic Ins
Ins Exothermic or endothermic
Exothermic or endothermic Exothermic vs endothermic
Exothermic vs endothermic Condensation particles
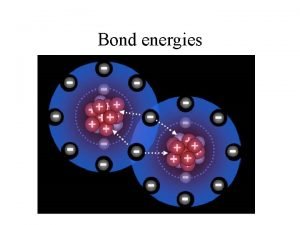
Condensation particles Bond energies
Bond energies Propane burning in a grill endothermic or exothermic
Propane burning in a grill endothermic or exothermic Endothermic vs exothermic graphs
Endothermic vs exothermic graphs Is a firecracker exploding endothermic or exothermic
Is a firecracker exploding endothermic or exothermic Where is the mitochondria located
Where is the mitochondria located Baking bread endothermic or exothermic
Baking bread endothermic or exothermic Endothermic process
Endothermic process Phase change diagram endothermic exothermic
Phase change diagram endothermic exothermic Exothermic reaction increase temperature equilibrium
Exothermic reaction increase temperature equilibrium In exothermic reaction temperature
In exothermic reaction temperature Exothermic reaction increase temperature equilibrium
Exothermic reaction increase temperature equilibrium Basic chemistry
Basic chemistry Chemical reaction examples
Chemical reaction examples Whats an exothermic reaction
Whats an exothermic reaction Exothermic reaction
Exothermic reaction Exothermic reaction
Exothermic reaction Unit rate vocabulary
Unit rate vocabulary Ratio guided notes
Ratio guided notes Ratios rates and unit rates
Ratios rates and unit rates Ratios rates and unit rates
Ratios rates and unit rates Physical property
Physical property Endothermic reaction for kids
Endothermic reaction for kids Specific heat formula
Specific heat formula Endothermic potential energy diagram
Endothermic potential energy diagram Mini unit reaction rates and equilibrium
Mini unit reaction rates and equilibrium Mini unit reaction rates and equilibrium
Mini unit reaction rates and equilibrium Reaction rates and equilibrium worksheet answers chapter 19
Reaction rates and equilibrium worksheet answers chapter 19 Section 4 reaction rates and equilibrium
Section 4 reaction rates and equilibrium Chapter 18 reaction rates and equilibrium answer key
Chapter 18 reaction rates and equilibrium answer key Chapter 18 reaction rates and equilibrium
Chapter 18 reaction rates and equilibrium Expressing reaction rates
Expressing reaction rates Rates of reaction quiz
Rates of reaction quiz Expressing reaction rates
Expressing reaction rates Did a chemical reaction occur
Did a chemical reaction occur Mixed flow reaction turbine
Mixed flow reaction turbine Pasadena water and power rates
Pasadena water and power rates Energy energy transfer and general energy analysis
Energy energy transfer and general energy analysis Energy energy transfer and general energy analysis
Energy energy transfer and general energy analysis Rate of reaction formula
Rate of reaction formula