Jeopardy GAME RULES Room split into 2 teams










































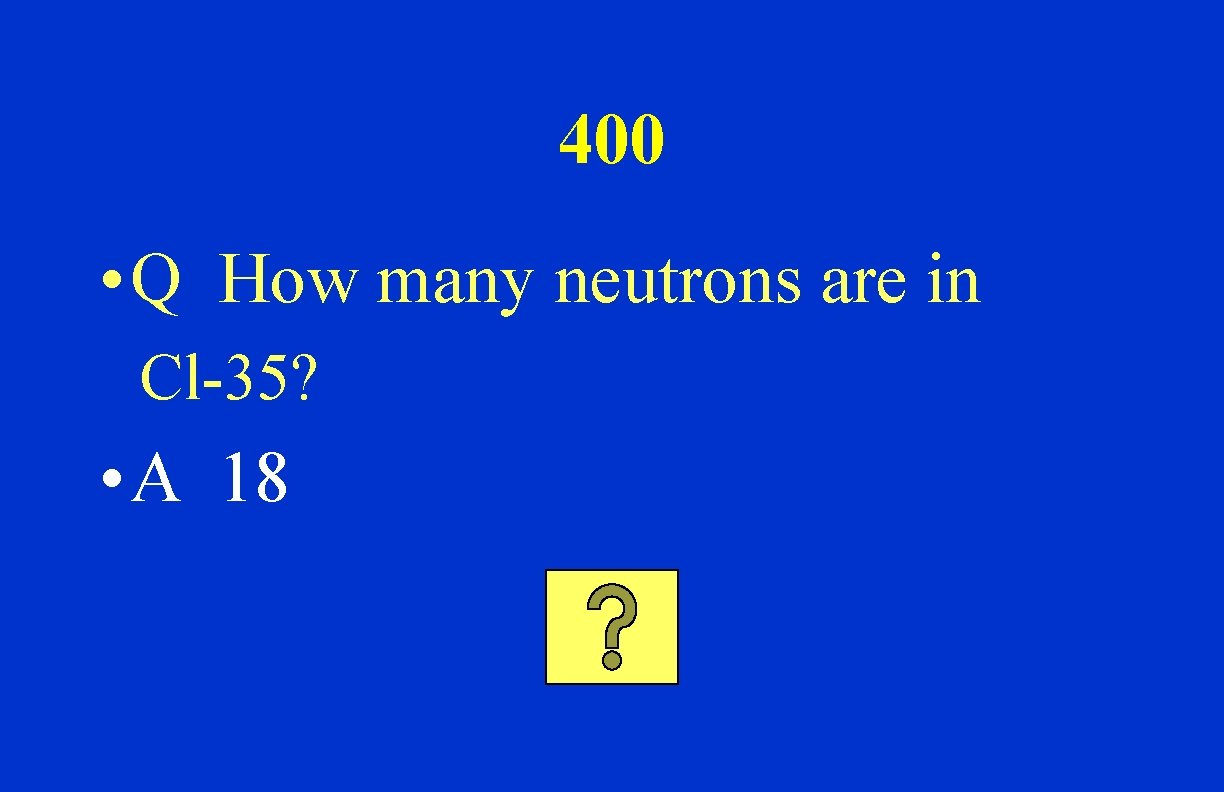
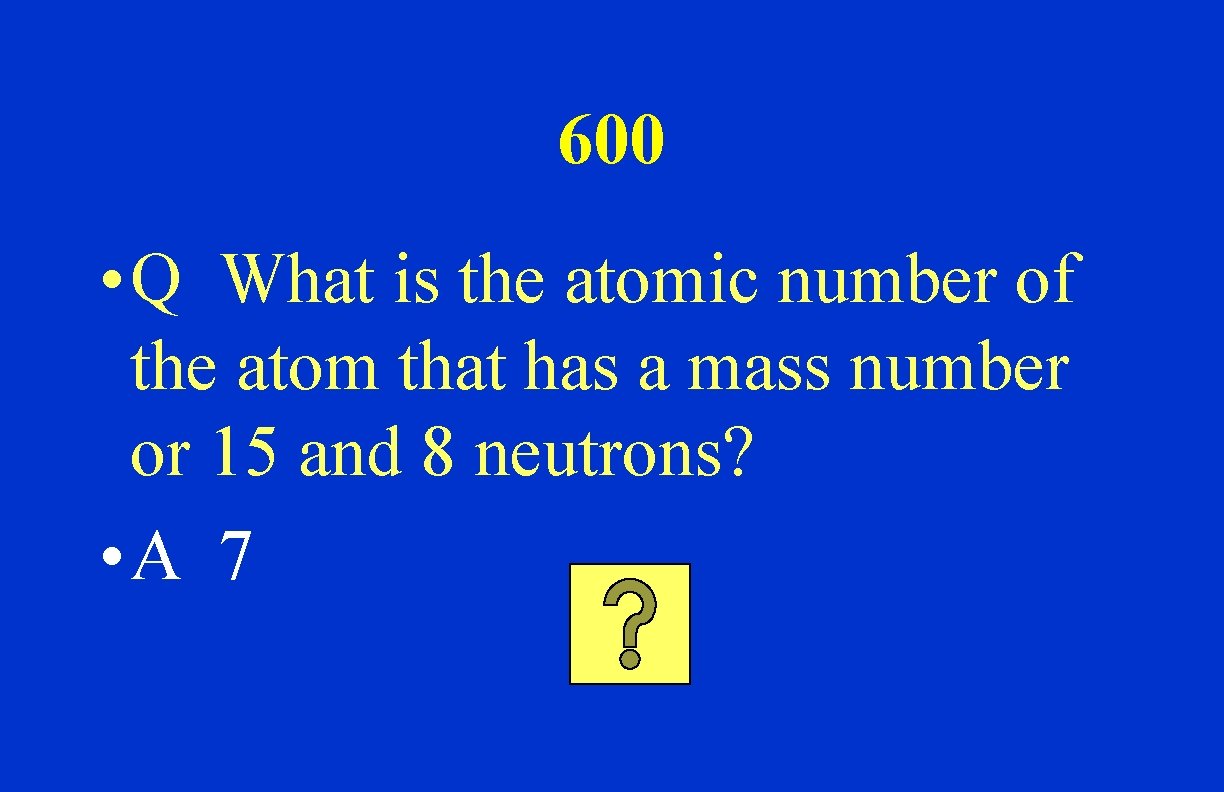

















- Slides: 68

Jeopardy

GAME RULES: • • Room split into 2 teams (RIGHT Vs. LEFT) Winner of the coin toss decides the first question Each team will have 1 person compete at a time. If the team answers incorrectly the other team has a chance to answer • If you think you know the answer raise your hand • The score will be kept on the board

ANY QUESTIONS?

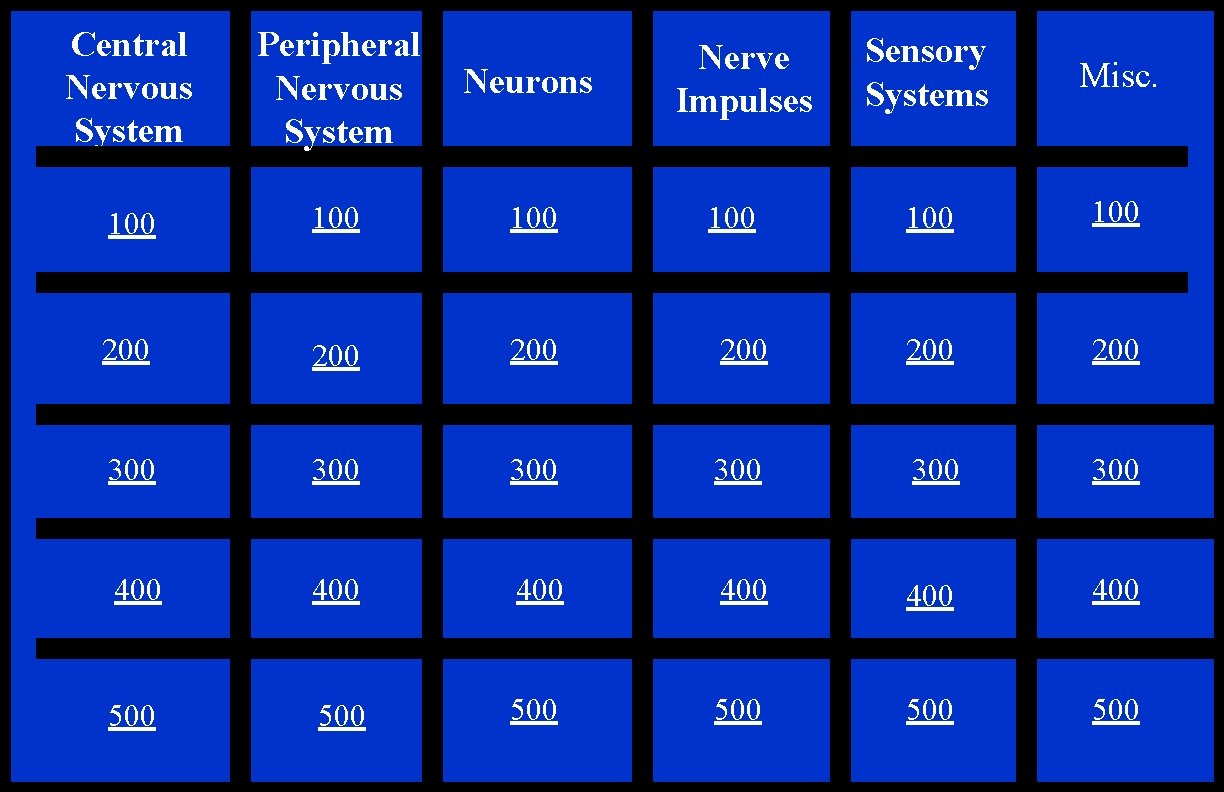
Central Nervous System Peripheral Nervous System Neurons 100 100 200 200 300 400 500 Sensory Systems Misc. 100 200 200 300 300 400 400 400 500 500 500 Nerve Impulses 100

The mouse has led you astray, please pick again!!

300 • Q What are the two main components of the central nervous system? • A Brain and Spinal Cord

200 • Q What is the largest part of the brain? • A The cerebrum

100 • Q Which structure in the central nervous system is responsible for balance? • A The cerebellum

400 • Q What are the two components of the brainstem? • A The pons and the medulla oblongata

500 • Q Which structure connects the right cerebral hemisphere with the left cerebral hemisphere • A The corpus callosum

100 • Q Which division of the peripheral nervous system controls involuntary body functions? • A Autonomic Nervous System

200 • Q Which division of the autonomic nervous system is responsible for preparing the body for action • A The sympathetic division

300 • Q Which division of the autonomic nervous system involves voluntary muscle action? • A The somatic nervous system

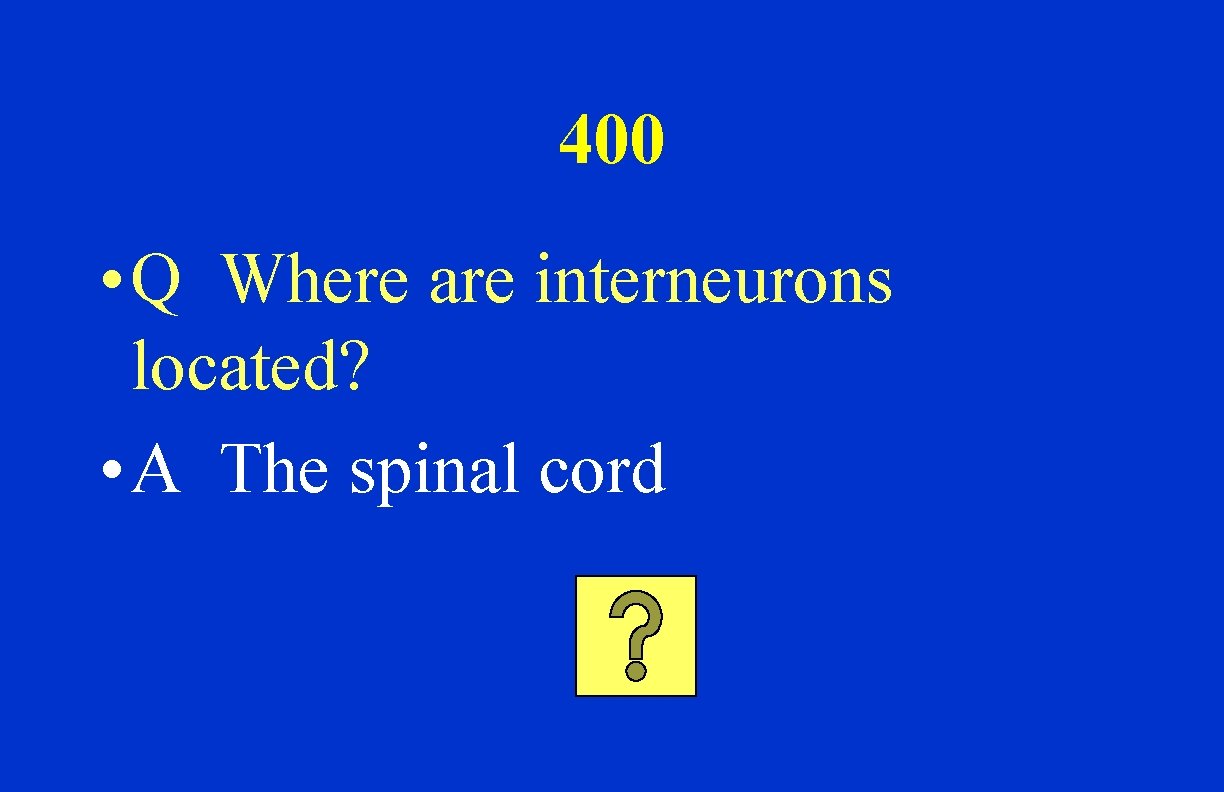
400 • Q Where are interneurons located? • A The spinal cord

100 • Q Which part of the neuron receives information? • A Dendrite

500 • Q Which three neurons are involved in a spinal reflex? • A Sensory neuron, interneuron, and motor neuron

200 • Q What part of the neuron transmits information? • A Axon

300 • Q What is the name of the junction where axons and dendrites meet? • A The synapse

400 • Q In what part of the neuron can the nucleus be found? • A Cell body

500 • Q What is the name of the layer of insulation that covers the axon in a neuron? • A Myelin Sheath

100 • Q What type of signal is transmitted in a neuron along its axon? • A Electrical

200 • Q At rest, what is the charge on the inside of the neuron? • A Negative

300 • Q What type of ion channels open up as a nerve impulse travels down an axon? A Sodium

400 • Q What type of signal is transmitted at the synapse? • A Chemical

500 • Q What process is involved in the release of neutransmitters at the synapse of a neuron? • A Exocytosis

100 • Q What type of receptors are sensitive to light? • A Photoreceptors

200 • Q What are the names of the two receptors in the retina of the eye that perceive light and color? • A Rods and cones

300 • Q What type of receptor is involved in both taste and smell? • A Chemoreceptors

400 • Q What type of receptors are stimulated by vibrations within the ear? • A Mechanoreceptors

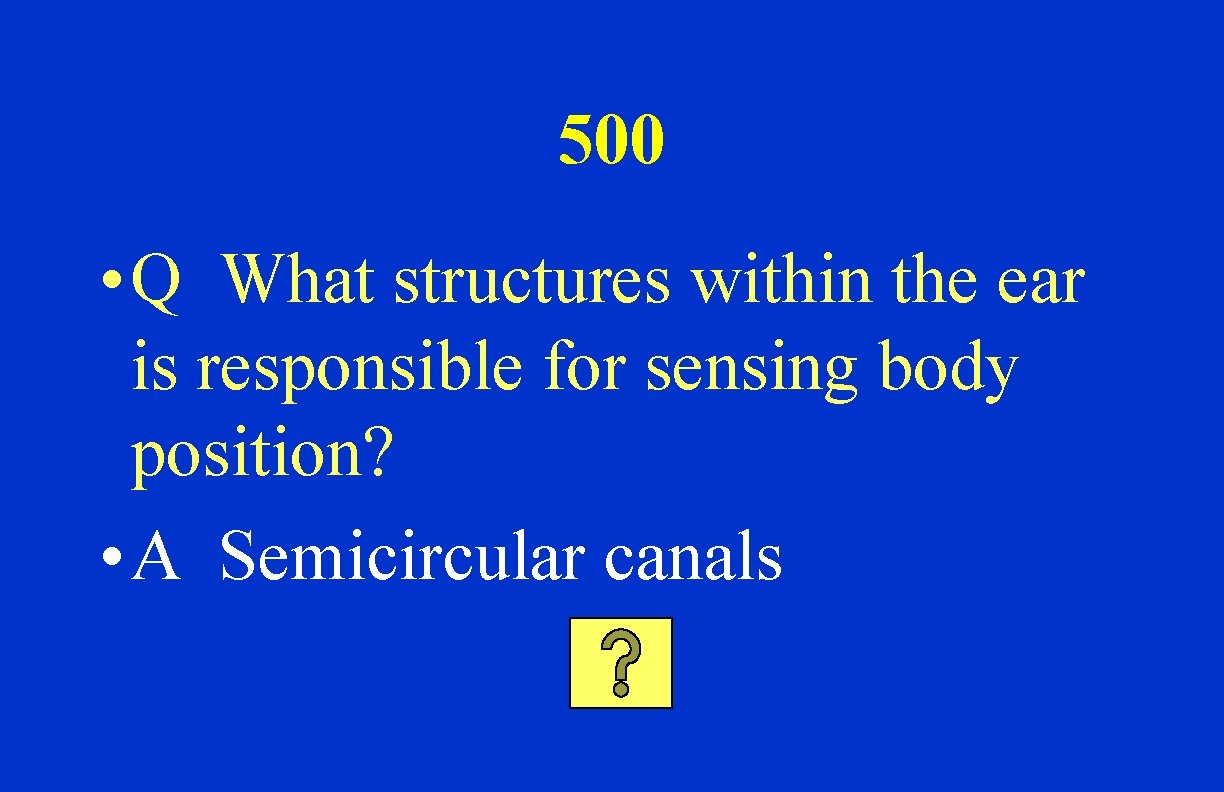
500 • Q What structures within the ear is responsible for sensing body position? • A Semicircular canals

100 • Q Which lobe of the brain is responsible of intellectual function? • A Frontal

200 • Q Which lobe of the brain processes information from the auditory nerve? • A Temporal

300 • Q What is an involuntary response to a stimulus? • A reflex

400 • Q To which lobe of the brain does the optic nerve travel? • A Occipital

500 • Q Which lobe of the brain is responsible for somatosensory processing? • A Parietal lobe

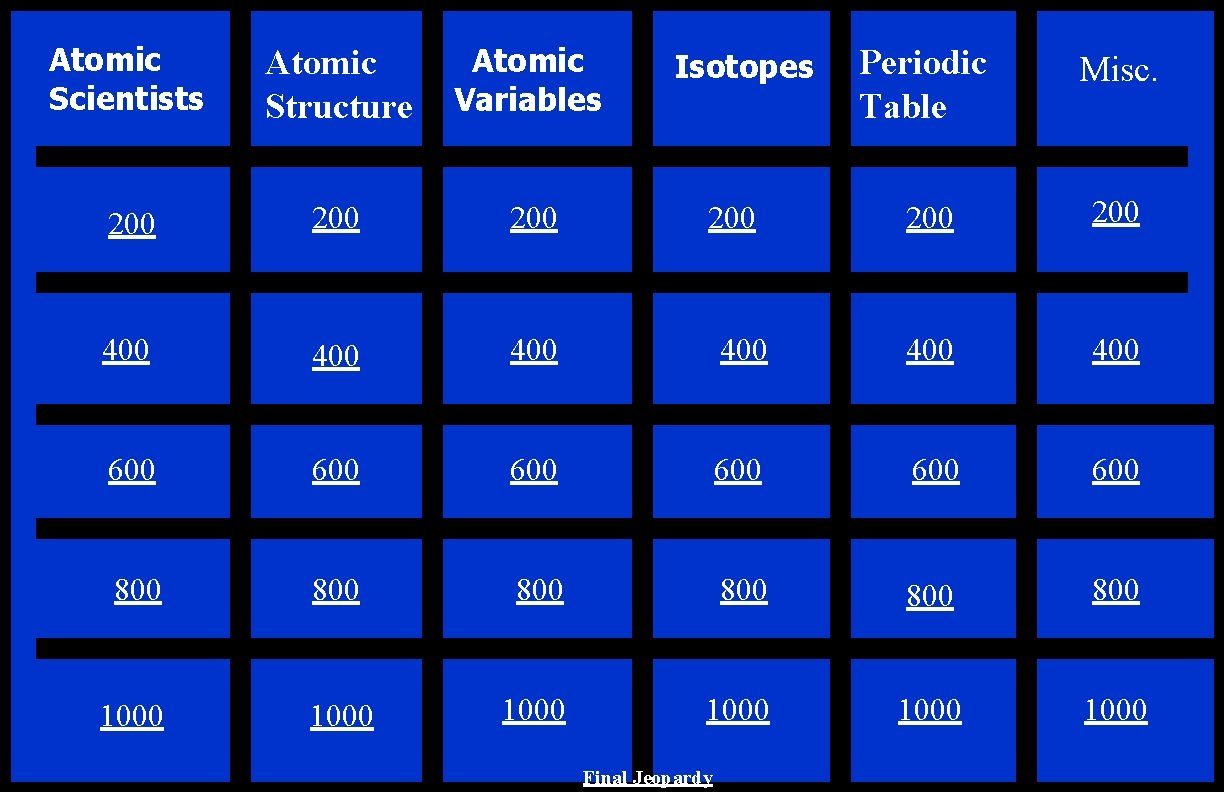
Atomic Scientists Atomic Structure Atomic Variables 200 200 400 400 600 800 1000 Periodic Table Misc. 200 400 400 600 600 800 800 800 1000 1000 Isotopes 200 Final Jeopardy

The mouse has led you astray, please pick again!!

200 • Q What was the name given to JJ Thomson’s atomic model? • A Plum Pudding

400 • Q According to John Dalton, Which atoms are identical? • A Atoms of the same element

600 • Q Who first proposed that all matter consisted of atoms? • A Democritus

800 • Q Who was responsible for describing the nuclear model of particles in the atom? • A Ernest Rutherford

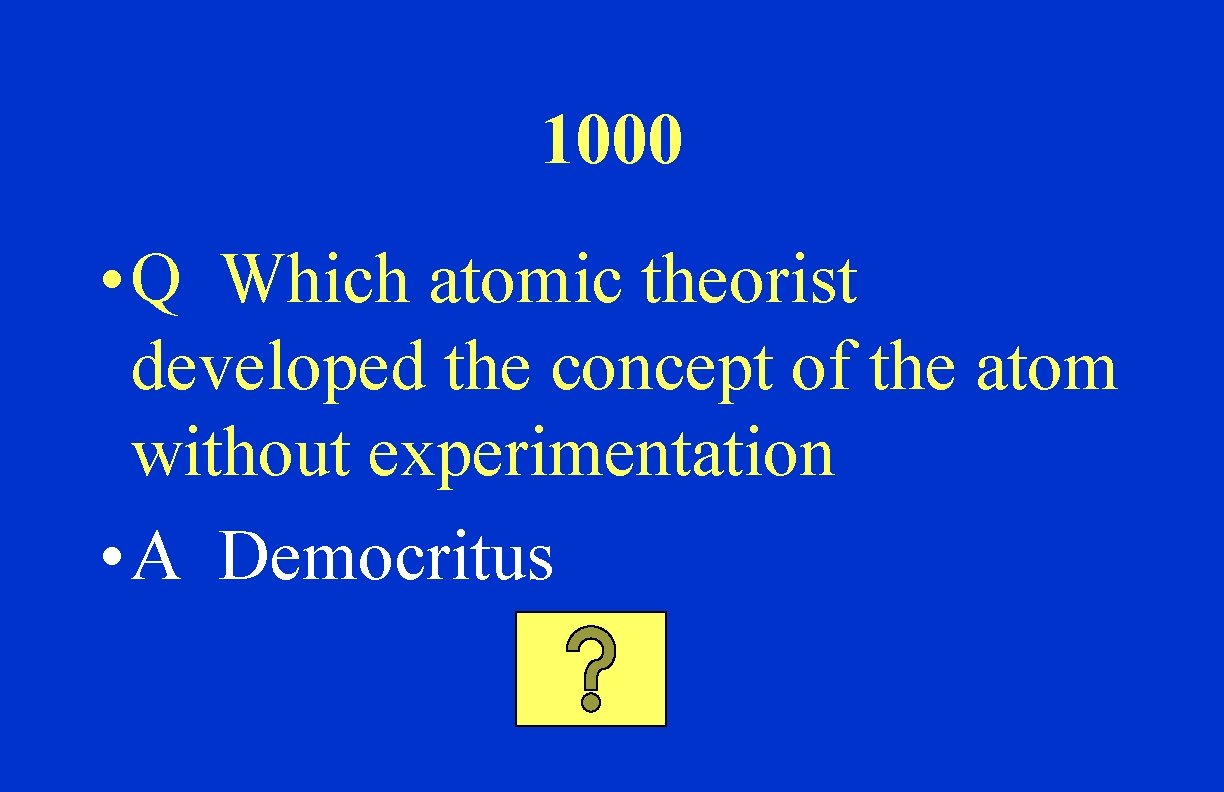
1000 • Q Which atomic theorist developed the concept of the atom without experimentation • A Democritus

200 • Q In terms of charge, how would you compare a proton and an electron? • A Equal and opposite

Misc. 400 • Q What is the relative mass of an electron? • A 1/1840

600 • Q What would occur if two electrons were brought together? • A They would repel one another

800 • Q What does a change in the number of electrons in an atom give rise to? • A Ions

1000 • Q Which particle, proton or electron, is attracted to the cathode of a CRT? • A Proton

200 • Q From what is atomic mass derived? • A The weighted average mass of all atoms that naturally occur in a sample
400 • Q How many neutrons are in Cl-35? • A 18
600 • Q What is the atomic number of the atom that has a mass number or 15 and 8 neutrons? • A 7

800 • Q How many electrons are there in a calcium ion? • A 18

1000 • Q What type of energy determines whether a fission or fusion reaction takes place • A Binding energy

200 • Q What isotope is used to determine the age of carbon based materials? • A C-14

400 • Q What is a radioactive isotope? • A An isotope that has an unstable nucleus and undergoes radioactive decay.

600 • Q What subatomic particle is beta radiation composed of? A electron

800 • Q Which isotope is more abundant? F-19 or F-22 given an atomic mass of 20. 2 A F-19

1000 • Q What type of decay would occur in an isotope that has 4 neutrons and 2 protons? • A Beta radiation

200 • Q Which type of radiation has the least penetrating power? • A Alpha

400 • Q How do the mass of a proton and the mass of a neuron compare? • A They are roughly the same

600 • Q What are alpha particles composed of? • A Positively charged helium ions

800 • What type of nuclear reaction occurs in small nuclei to bring about stability • A fusion

1000 • Q What type of particles caused maximum deflection of alpha particles in Rutherford’s experiment? • A Protons

200 • Q Changes in the number of these particles result in ions? • A electrons

400 • Q What equation accounts for the conversion of mass into energy in nuclear reactions 2 • A E=mc

600 • Q What type of particles did Rutherford use in his experiment? A Alpha particles

800 • Q What subatomic particle was discovered by inference to account for the discrepancy between particle mass and atomic mass? • A Neutron

1000 • Q Which type of radiation would not be deflected by a magnet? • A Gamma

Game Over