JCTLM REFERENCE LABORATORY SERVICES Report of WG 2

JCTLM REFERENCE LABORATORY SERVICES Report of WG 2 November 14, 2005 _____________________

Joint Committee for Traceability in Laboratory Medicine ______________________________________________________ Working Group I: - Reference Materials, - Reference Measurement Procedures Working Group II: - Reference Measurement Laboratories

Joint Committee for Traceability in Laboratory Medicine ___________________________ Declaration of Cooperation Appendix III: … The technical competence of the laboratories shall be demonstrated by their performance in international comparisons, and their operation of an appropriate quality system. International recognition of the implementation of the quality system is achieved via accreditation or equivalent documented peer review. …

Joint Committee for Traceability in Laboratory Medicine ___________________________ Working Group II Objectives: - to prepare a procedure manual describing the processes for nomination, listing and de-listing of reference laboratory services, - to publish an open call for nomination of reference laboratory services

Joint Committee for Traceability in Laboratory Medicine ___________________________ Working Group II Procedure Manual JCTLM WG 2 P-00: Outline of the Calibration and Measurement Hierarchy in Laboratory Medicine Quality Policy and Definitions
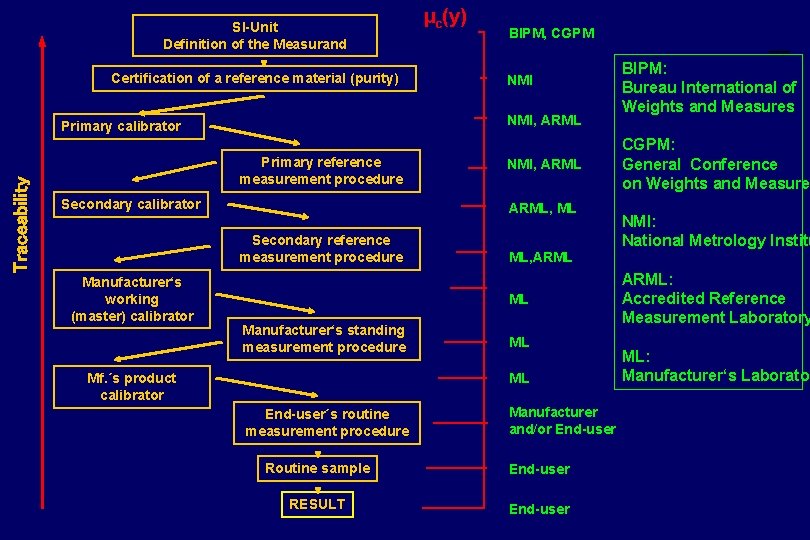
SI-Unit Definition of the Measurand Certification of a reference material (purity) Traceability BIPM, CGPM NMI, ARML Primary calibrator Primary reference measurement procedure Secondary calibrator NMI, ARML, ML Secondary reference measurement procedure Manufacturer‘s working (master) calibrator µc(y) ML, ARML ML Manufacturer‘s standing measurement procedure Mf. ´s product calibrator ML ML End-user´s routine measurement procedure Manufacturer and/or End-user Routine sample End-user RESULT End-user BIPM: Bureau International of Weights and Measures CGPM: General Conference on Weights and Measure NMI: National Metrology Institu ARML: Accredited Reference Measurement Laboratory ML: Manufacturer‘s Laborator

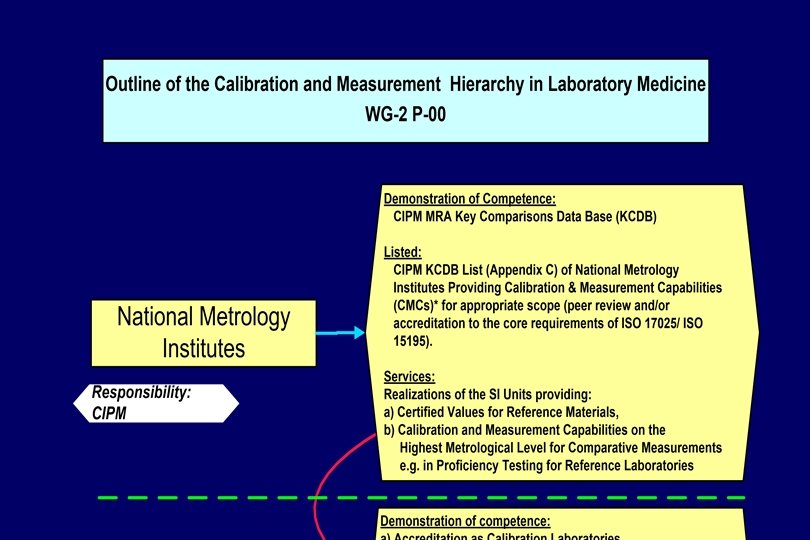
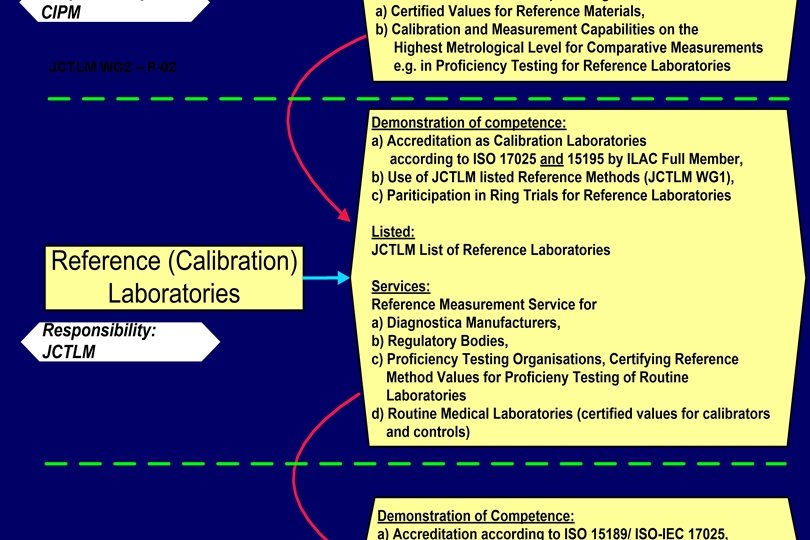
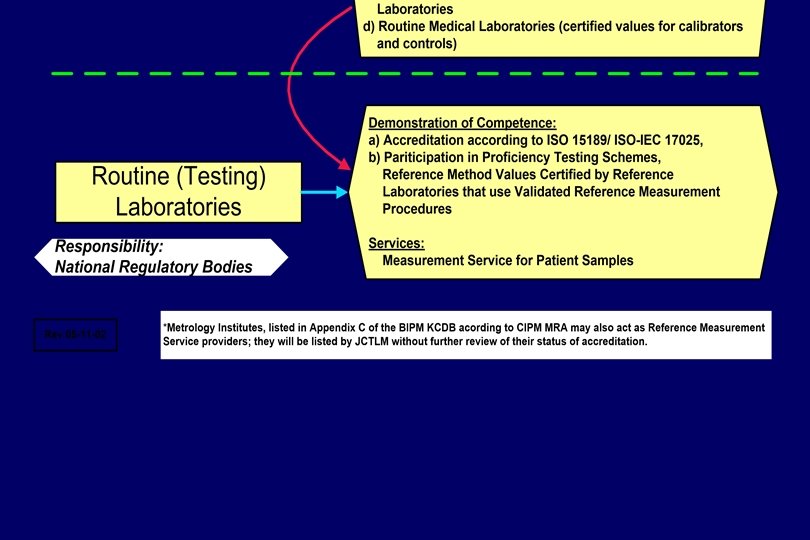
Hierarchy of Laboratories National Metrology Institutes Accredited Reference (Calibration) Laboratories (universities, hospitals, manufacturers) Routine (Testing) Laboratories

JCTLM WG 2 – P-02

Joint Committee for Traceability in Laboratory Medicine ___________________________ Working Group II Procedure Manual JCTLM WG 2 P-01: Outline of the JCTLM Procedures for Identifying and Listing Reference Measurement Services Provided by Reference Laboratories
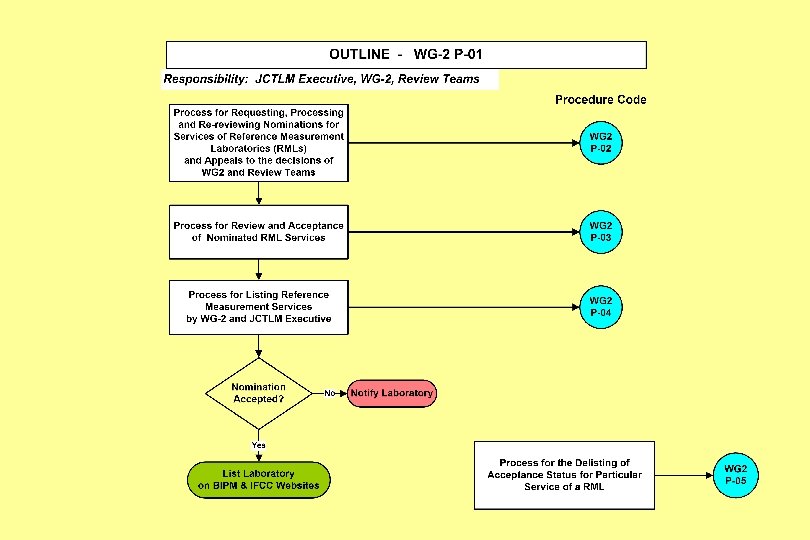

Joint Committee for Traceability in Laboratory Medicine ___________________________ Working Group II Procedure Manual JCTLM WG 2 P-02: Process for Requesting and Processing Nominations for JCTLM Listing of Reference Measurement Services
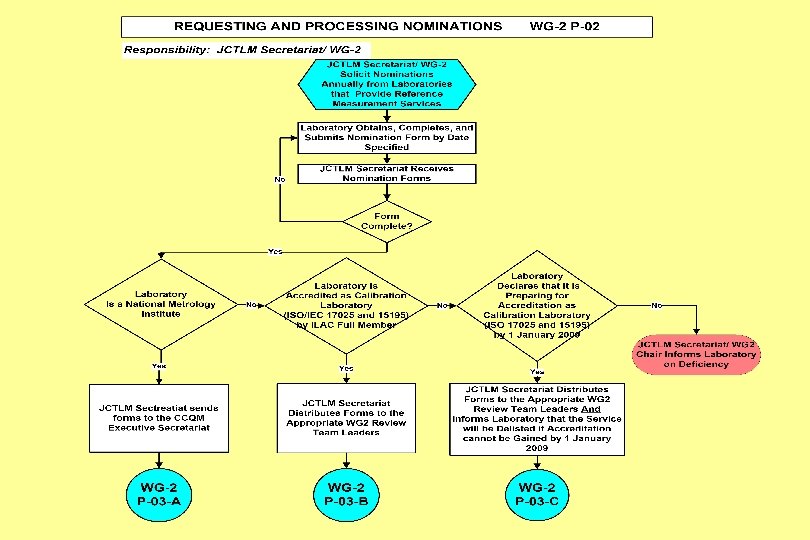

JCTLM WG 2 – P-02 7. Procedure 7. 1 The process for nominating and reviewing RML services for recommendation by the JCTLM for listing will occur annually (see flow chart in the Attachment). 7. 2 Solicitation of Nominations from RMLs wishing their services to be listed on the JCTLM List: 7. 3 Obtaining Nomination Forms: 7. 4 Submitting Nomination Forms for Consideration by WG 2. 7. 5 Preliminary Review of submitted nominations for completeness by the JCTLM Secretariat. 7. 6 The JCTLM Secretariat and the WG 2 Chair or the Chair’s designee review nominations within six weeks of their receipt. 7. 7 Distributing Nominations from National Metrology Institutes to the JCTLM/CCQM Executive Secretariat. 7. 8 Distributing Nominations from Accredited Laboratories (or Laboratories preparing for Accreditation) to Review Teams. 7. 9 Any Review Team member affiliated to a nominating organization will not participate in the evaluation of that reference measurement laboratory service if a potential conflict of interest exists or is perceived to exist.

JCTLM WG 2 – P-02 7. Procedure (continued) 7. 10 The distributed nominations from National Metrology Institutes are verified by the JCTLM/CCQM Secretariat. 7. 11 The distributed nominations of Reference Measurement Service providers are reviewed by the Review Teams. 7. 12 For laboratories where accreditation is pending (7. 7. 1. 2) the status of accreditation is re-examined in 2009 by the JCTLM Secretariat after consulting WG 2 chair. 7. 13 Reference measurement services listed on the JCTLM website will be re-reviewed every three years. 7. 14 Any appeal against decisions of WG 2 chair or review teams will be submitted to the Secretariat and finally decided by the JCTLM Executive after consulting the WG 2 chair.
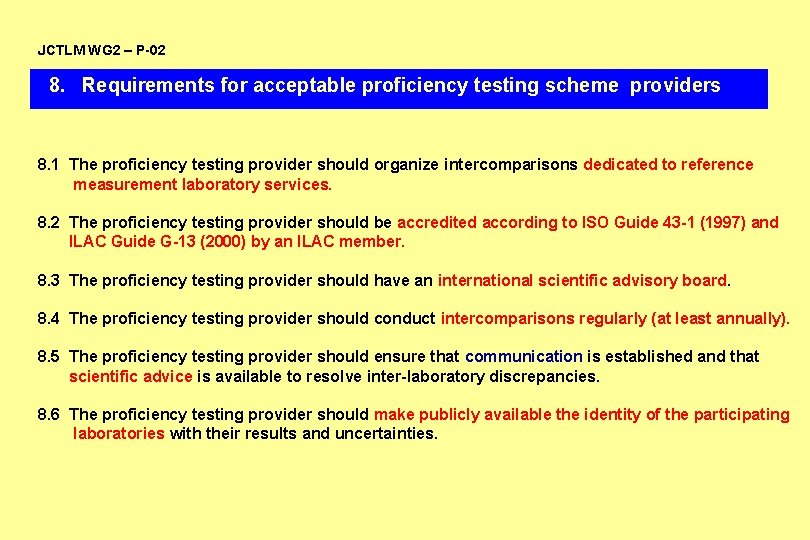
JCTLM WG 2 – P-02 8. Requirements for acceptable proficiency testing scheme providers 8. 1 The proficiency testing provider should organize intercomparisons dedicated to reference measurement laboratory services. 8. 2 The proficiency testing provider should be accredited according to ISO Guide 43 -1 (1997) and ILAC Guide G-13 (2000) by an ILAC member. 8. 3 The proficiency testing provider should have an international scientific advisory board. 8. 4 The proficiency testing provider should conduct intercomparisons regularly (at least annually). 8. 5 The proficiency testing provider should ensure that communication is established and that scientific advice is available to resolve inter-laboratory discrepancies. 8. 6 The proficiency testing provider should make publicly available the identity of the participating laboratories with their results and uncertainties.

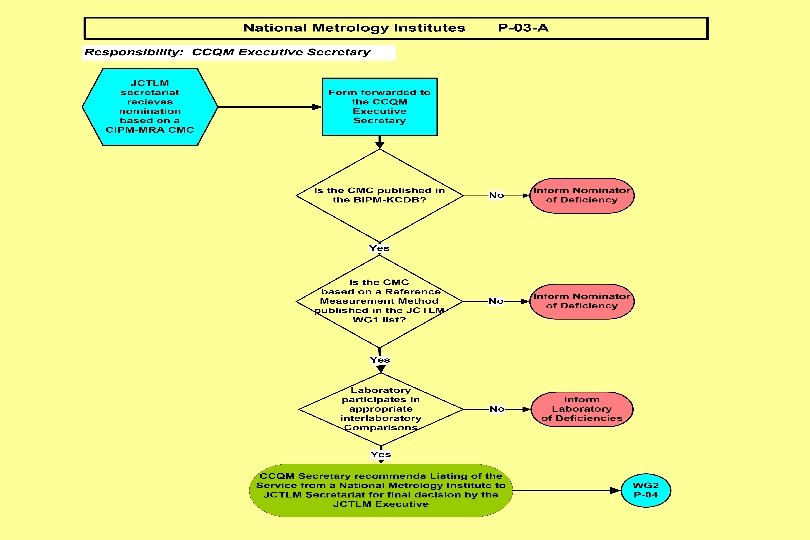
Joint Committee for Traceability in Laboratory Medicine ___________________________ Working Group II Procedure Manual JCTLM WG 2 P-03 -A: Process for Verification and Publication of Calibration and Measurement Capability (CMC) Claims in the JCTLM Database (National Metrology Institutes)

Joint Committee for Traceability in Laboratory Medicine ___________________________ Working Group II Procedure Manual JCTLM WG 2 P-03 -B: Process for Review of Reference Measurement Services from Laboratories that are Accredited as Calibration Laboratories (ISO/IEC 17025 and ISO 15195)
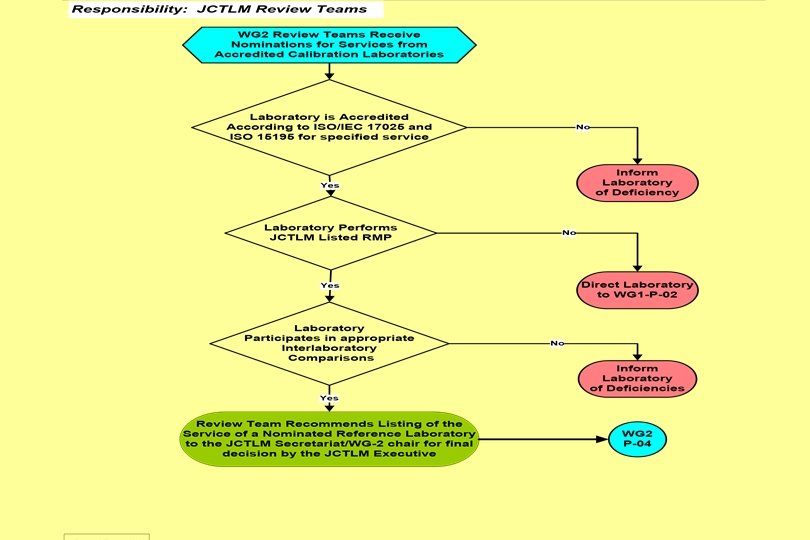
JCTLM WG 2 – P-03 -B 7. Procedure 7. 1 The process for reviewing RML services by the Review Teams will take place annually. WG 2 chair will send documentation from the candidate RMLs to the review teams. They will assess the documentation and other publicly available information according to the following criteria: 7. 1. 1 Review teams ascertain that the RML is accredited according to ISO/IEC 17025 and ISO 15195 as calibration laboratory including on-site assessment by ILAC full members on the basis of on-site assessments by technical and management experts from accreditation bodies which are ILAC full members. 7. 1. 2 Review teams ascertain whether the Reference Method applied by the candidate laboratory is listed by the JCTLM according to the WG 1 procedure. If the applied method has not yet been included in the WG 1 reference method list the laboratory will be notified to nominate the procedure for acceptance by JCTLM WG 1. 7. 1. 3 Review teams ascertain regular (at least annual) participation of the candidate laboratory in inter laboratory comparisons provided by an appropriate proficiency testing/external quality assessment scheme for reference laboratories which fulfills the requirements of WG-2 P-02 -8. The results of the inter-comparisons as well as the result of the individual laboratory related to its identity shall be publicly available. It is also acceptable that a laboratory participates for another measurand of the same group of quantities (e. g. , metabolites & substrates, low-molecular hormones, therapeutic drugs, enzymes) provided the same principle of measurement - e. g. , IDMS, kinetic spectrophotometry for enzyme activities - is applied.

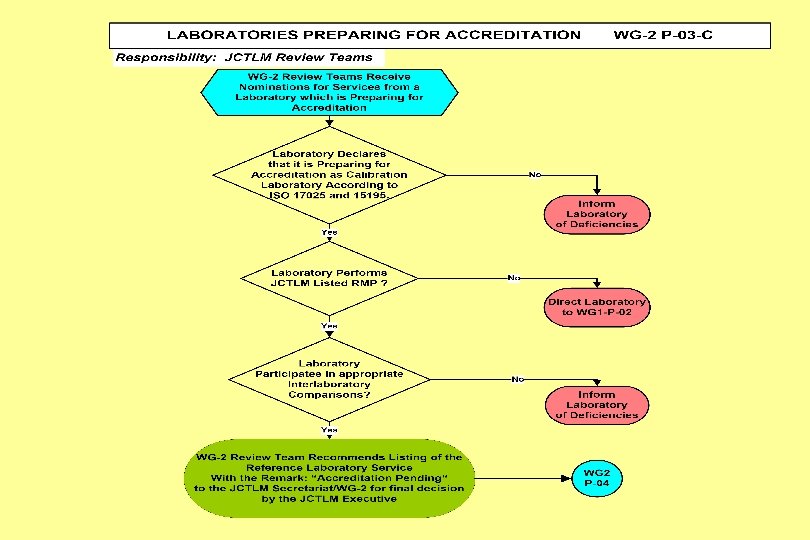
Joint Committee for Traceability in Laboratory Medicine ___________________________ Working Group II Procedure Manual JCTLM WG 2 P-03 -C: Process for Review of Reference Measurement Services from Laboratories Preparing for Accreditation

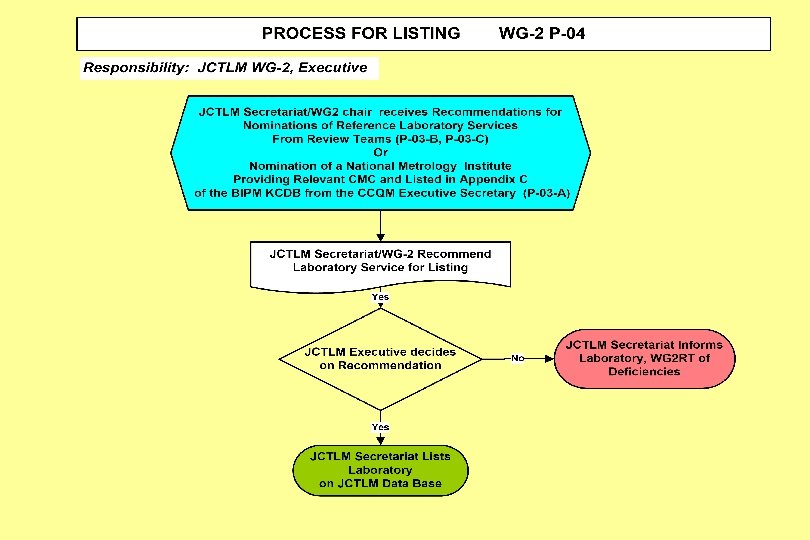
Joint Committee for Traceability in Laboratory Medicine ___________________________ Working Group II Procedure Manual JCTLM WG 2 P-04: Process for Listing Reference Measurement Laboratory Services

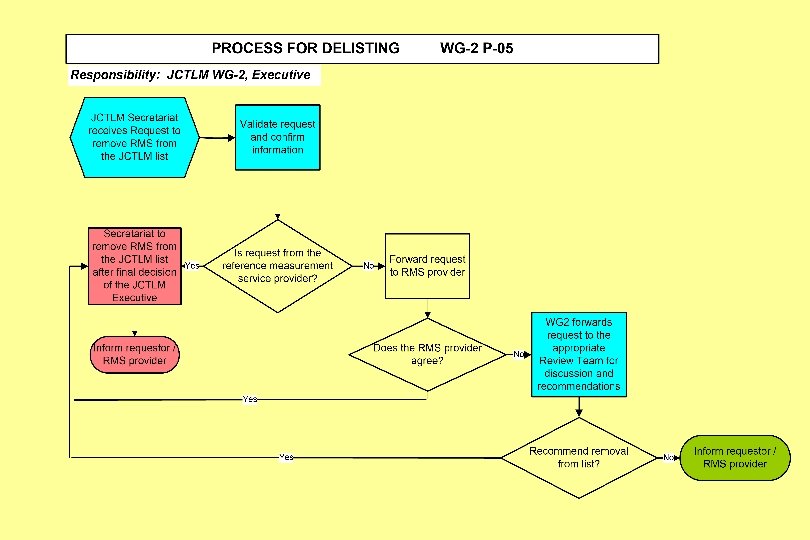
Joint Committee for Traceability in Laboratory Medicine ___________________________ Working Group II Procedure Manual: JCTLM WG 2 P-05: Process for Delisting Reference Measurement Laboratory Services

Reference Measurement Laboratories will be identified - according to the metrological level of the reference procedures applied; JCTLM –listed WG 1, - on the basis of accreditation according to ISO 17025 and including on-site ISO 15195 as reference/calibration laboratory assessments by ILAC full members (signatories); or NMI listed in Appendix C of the CIPM MRA (peer reviewed), - on the basis of their ability to demonstrate in regular (at least annual) intertrials). performance laboratory comparisons (ring
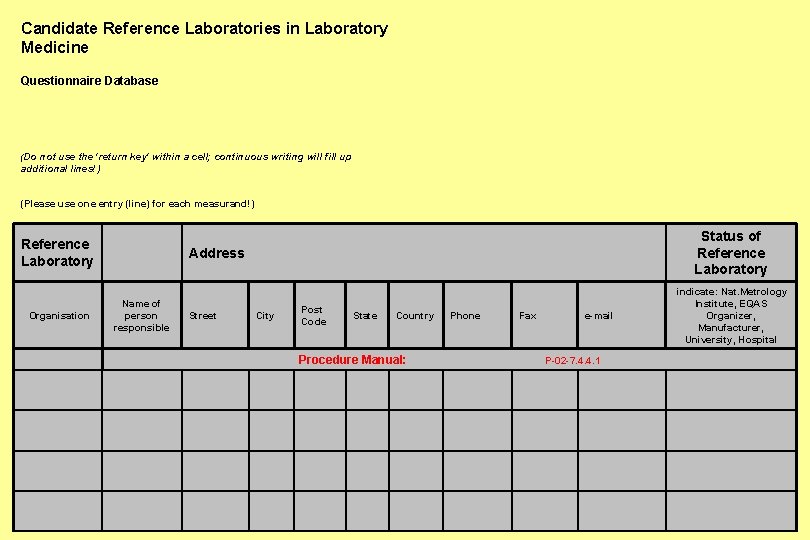
Candidate Reference Laboratories in Laboratory Medicine Questionnaire Database (Do not use the 'return key' within a cell; continuous writing will fill up additional lines!) (Please use one entry (line) for each measurand!) Reference Laboratory Status of Reference Laboratory Address Name of Organisation person Street responsible City Post Code State Country Phone Fax e-mail indicate: Nat. Metrology Institute, EQAS Organizer, Manufacturer, University, Hospital Procedure Manual: P-02 -7. 4. 4. 1
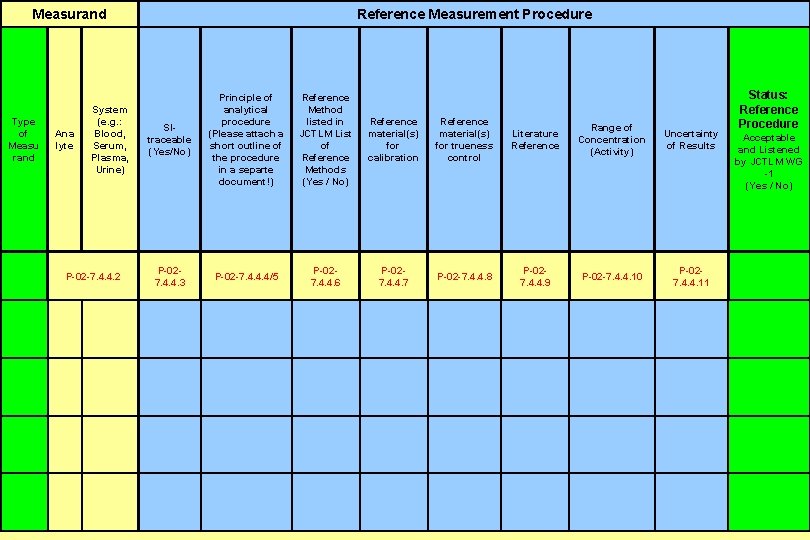
Measurand Principle of Reference System analytical Method (e. g. : procedure listed in Reference SIAna Blood, (Please attach a JCTLM List material(s) traceable lyte Serum, short outline of of for (Yes/No) Plasma, the procedure Reference calibration Urine) in a separte Methods document!) (Yes / No) Type of Measu rand Reference Measurement Procedure P-027. 4. 4. 3 P-02 -7. 4. 4. 2 P-027. 4. 4. 6 P-02 -7. 4. 4. 4/5 P-027. 4. 4. 7 Reference material(s) for trueness control P-027. 4. 4. 9 P-027. 4. 4. 11 P-02 -7. 4. 4. 10 Range of Literature Uncertainty Acceptable Concentration Reference of Results and Listened (Activity) by JCTLM WG -1 (Yes / No) P-02 -7. 4. 4. 8 Status: Reference Procedure
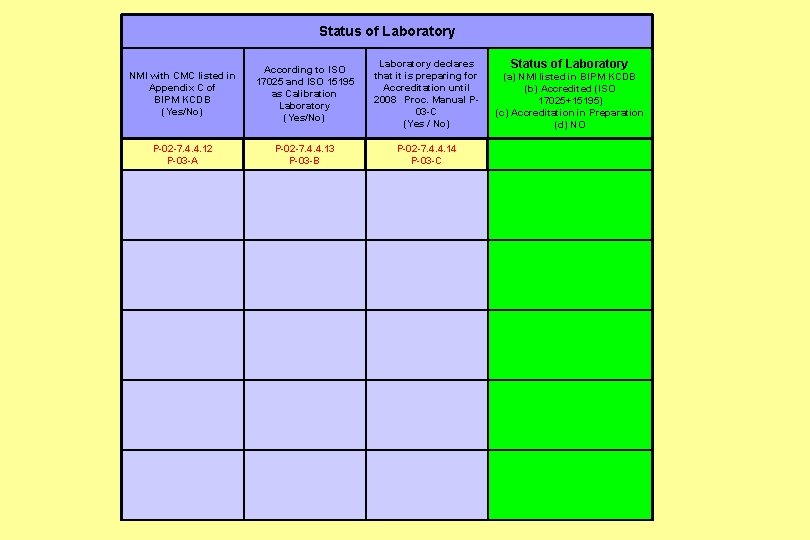
Status of Laboratory declares Status of Laboratory According to ISO NMI with CMC listed in that it is preparing for (a) NMI listed in BIPM KCDB 17025 and ISO 15195 Appendix C of Accreditation until (b) Accredited (ISO as Calibration BIPM KCDB 2008 Proc. Manual P 17025+15195) Laboratory (Yes/No) 03 -C (c) Accreditation in Preparation (Yes/No) (Yes / No) (d) NO P-02 -7. 4. 4. 12 P-02 -7. 4. 4. 13 P-02 -7. 4. 4. 14 P-03 -A P-03 -B P-03 -C
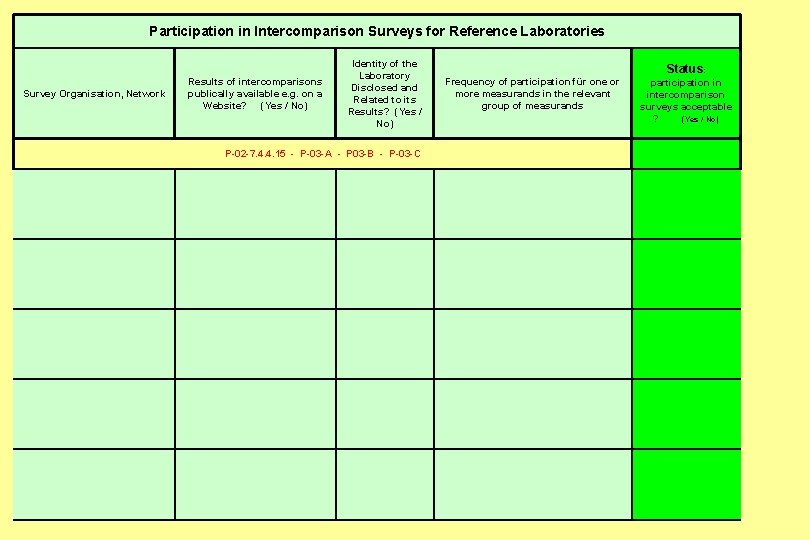
Participation in Intercomparison Surveys for Reference Laboratories Identity of the Laboratory Disclosed and Related to its Results? (Yes / No) Results of intercomparisons publically available e. g. on a Website? (Yes / No) Survey Organisation, Network Status: Frequency of participation für one or participation in more measurands in the relevant intercomparison group of measurands surveys acceptable ? (Yes / No) P-02 -7. 4. 4. 15 - P-03 -A - P 03 -B - P-03 -C
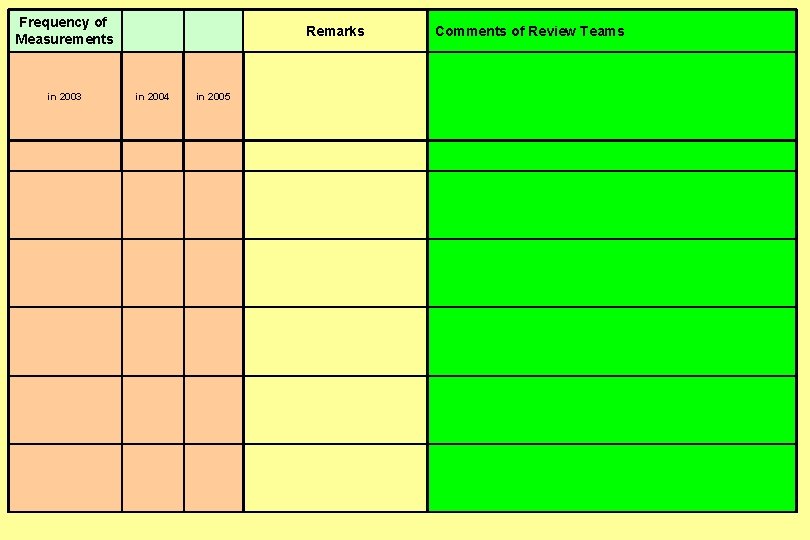
Frequency of Measurements Comments of Review Teams in 2003 in 2004 in 2005 Remarks
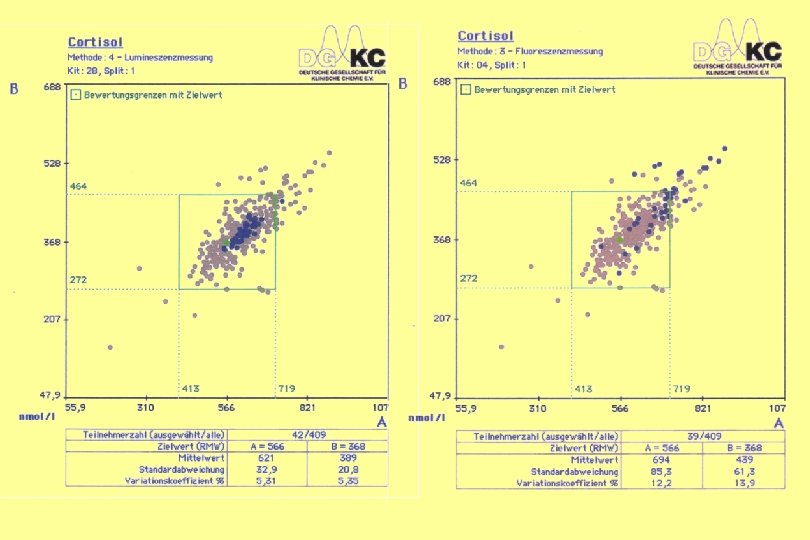
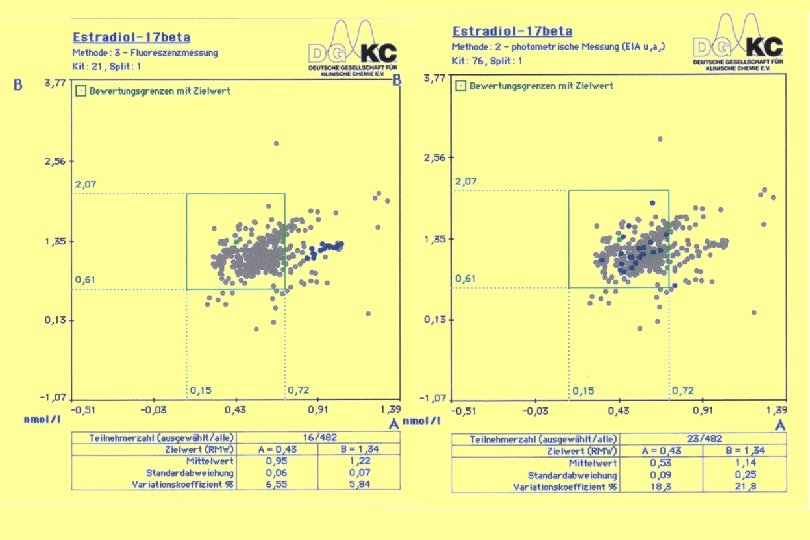

17 -OH-Progesterone Ring Trial HM 4/2004 Test Kit 111
- Slides: 39