JAVELIN Bladder 100 Phase III Trial of Maintenance
JAVELIN Bladder 100: Phase III Trial of Maintenance Avelumab After First-line Platinum Chemotherapy in Advanced Urothelial Carcinoma CCO Independent Conference Coverage* Highlights of the 2020 ASCO Virtual Scientific Meeting, May 29 -31, 2020 *CCO is an independent medical education company that provides state-of-the-art medical information to healthcare professionals through conference coverage and other educational programs. This activity is provided by Clinical Care Options, LLC Supported by educational grants from Astra. Zeneca; Bayer Healthcare Pharmaceuticals, Inc. ; Bristol-Myers Squibb; Exelixis; and Pfizer and EMD Serono, Inc.
About These Slides § Please feel free to use, update, and share some or all of these slides in your noncommercial presentations to colleagues or patients § When using our slides, please retain the source attribution: Slide credit: clinicaloptions. com § These slides may not be published, posted online, or used in commercial presentations without permission. Please contact permissions@clinicaloptions. com for details
JAVELIN Bladder 100: Background § First-line, platinum-based chemotherapy controls advanced urothelial carcinoma in 65% to 75% of cases, but PFS and OS limited due to development of chemotherapy resistance [1, 2] § Only 25% to 55% of patients go on to receive second-line therapy[3, 4] ‒ PD-1/PD-L 1 inhibitors, including avelumab, represent standard second-line treatment options [5 -7] ‒ Outcomes typically poor to due to rapid disease progression, with a minority of patients attaining durable clinical benefit[3 -7] § Use of PD-1/PD-L 1 inhibitors in the maintenance setting after response to first-line, platinum-based chemotherapy may enhance clinical benefit by allowing immunotherapy to sustain disease control in the absence of large tumor burden[8] § Current phase III trial evaluated efficacy of maintenance avelumab + BSC vs BSC alone in patients with advanced urothelial carcinoma without disease progression after first-line, platinum-based chemotherapy[9] 1. De Santis. JCO. 2012; 30: 191. 2. Dogliotti. Eur Urol. 2007; 52: 134. 3. Aly. J Med Econ. 2019; 22: 662. 4. Flannery. Future Oncol. 2019; 15: 1323. 5. Powles. Lancet. 2018; 391: 748. 6. Bellmunt. NEJM. 2017; 376: 1015. 7. Patel. Lancet Oncol. 2017; 18: 312. 8. Grivas. Target Oncol. 2019; 14: 505. 9. Powles. ASCO 2020. Abstr LBA 1. Slide credit: clinicaloptions. com
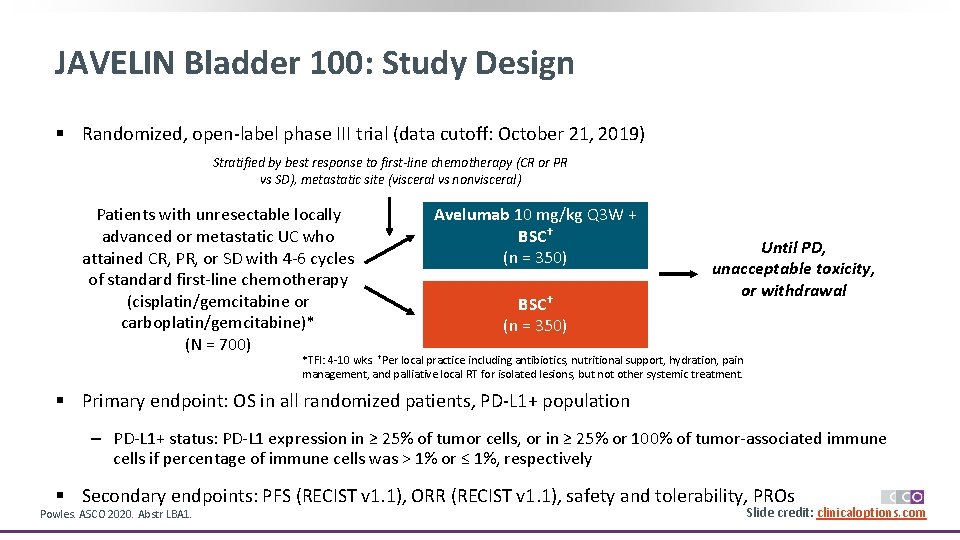
JAVELIN Bladder 100: Study Design § Randomized, open-label phase III trial (data cutoff: October 21, 2019) Stratified by best response to first-line chemotherapy (CR or PR vs SD), metastatic site (visceral vs nonvisceral) Patients with unresectable locally advanced or metastatic UC who attained CR, PR, or SD with 4 -6 cycles of standard first-line chemotherapy (cisplatin/gemcitabine or carboplatin/gemcitabine)* (N = 700) Avelumab 10 mg/kg Q 3 W + BSC† (n = 350) Until PD, unacceptable toxicity, or withdrawal *TFI: 4 -10 wks. †Per local practice including antibiotics, nutritional support, hydration, pain management, and palliative local RT for isolated lesions, but not other systemic treatment. § Primary endpoint: OS in all randomized patients, PD-L 1+ population ‒ PD-L 1+ status: PD-L 1 expression in ≥ 25% of tumor cells, or in ≥ 25% or 100% of tumor-associated immune cells if percentage of immune cells was > 1% or ≤ 1%, respectively § Secondary endpoints: PFS (RECIST v 1. 1), ORR (RECIST v 1. 1), safety and tolerability, PROs Powles. ASCO 2020. Abstr LBA 1. Slide credit: clinicaloptions. com
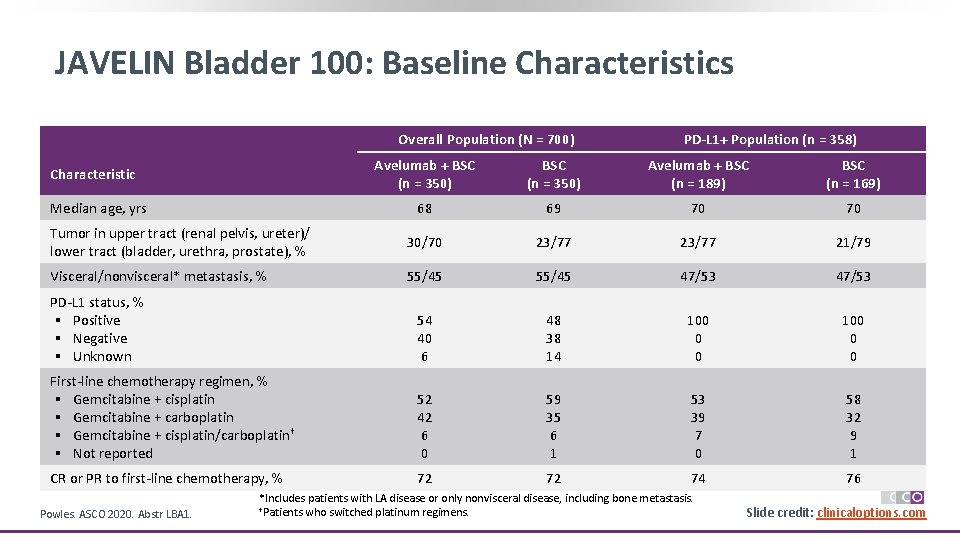
JAVELIN Bladder 100: Baseline Characteristics Overall Population (N = 700) PD-L 1+ Population (n = 358) Avelumab + BSC (n = 350) Avelumab + BSC (n = 189) BSC (n = 169) 68 69 70 70 Tumor in upper tract (renal pelvis, ureter)/ lower tract (bladder, urethra, prostate), % 30/70 23/77 21/79 Visceral/nonvisceral* metastasis, % 55/45 47/53 PD-L 1 status, % § Positive § Negative § Unknown 54 40 6 48 38 14 100 0 0 First-line chemotherapy regimen, % § Gemcitabine + cisplatin § Gemcitabine + carboplatin § Gemcitabine + cisplatin/carboplatin† § Not reported 52 42 6 0 59 35 6 1 53 39 7 0 58 32 9 1 CR or PR to first-line chemotherapy, % 72 72 74 76 Characteristic Median age, yrs Powles. ASCO 2020. Abstr LBA 1. *Includes patients with LA disease or only nonvisceral disease, including bone metastasis. †Patients who switched platinum regimens. Slide credit: clinicaloptions. com
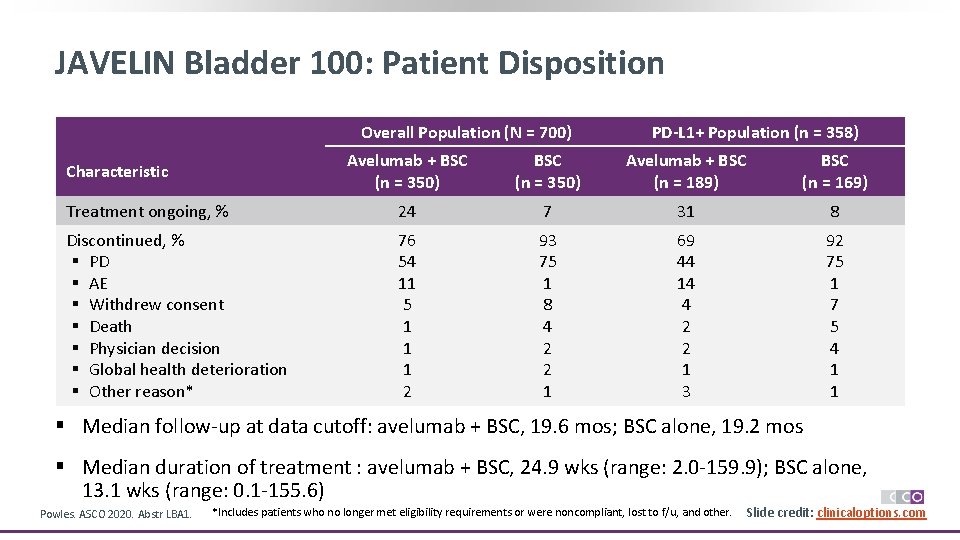
JAVELIN Bladder 100: Patient Disposition Overall Population (N = 700) PD-L 1+ Population (n = 358) Avelumab + BSC (n = 350) Avelumab + BSC (n = 189) BSC (n = 169) Treatment ongoing, % 24 7 31 8 Discontinued, % § PD § AE § Withdrew consent § Death § Physician decision § Global health deterioration § Other reason* 76 54 11 5 1 1 1 2 93 75 1 8 4 2 2 1 69 44 14 4 2 2 1 3 92 75 1 7 5 4 1 1 Characteristic § Median follow-up at data cutoff: avelumab + BSC, 19. 6 mos; BSC alone, 19. 2 mos § Median duration of treatment : avelumab + BSC, 24. 9 wks (range: 2. 0 -159. 9); BSC alone, 13. 1 wks (range: 0. 1 -155. 6) Powles. ASCO 2020. Abstr LBA 1. *Includes patients who no longer met eligibility requirements or were noncompliant, lost to f/u, and other. Slide credit: clinicaloptions. com
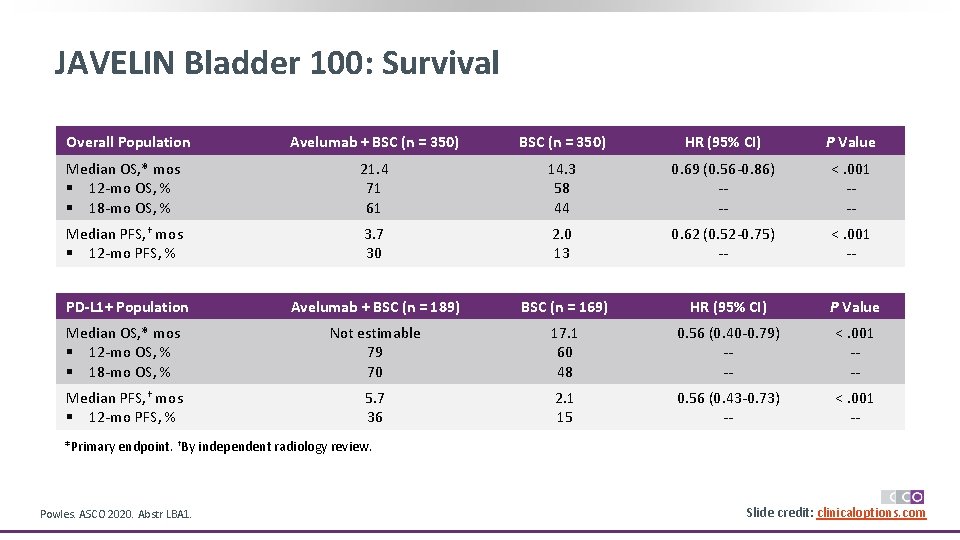
JAVELIN Bladder 100: Survival Overall Population Avelumab + BSC (n = 350) HR (95% CI) P Value Median OS, * mos § 12 -mo OS, % § 18 -mo OS, % 21. 4 71 61 14. 3 58 44 0. 69 (0. 56 -0. 86) --- <. 001 --- Median PFS, † mos § 12 -mo PFS, % 3. 7 30 2. 0 13 0. 62 (0. 52 -0. 75) -- <. 001 -- PD-L 1+ Population Avelumab + BSC (n = 189) BSC (n = 169) HR (95% CI) P Value Median OS, * mos § 12 -mo OS, % § 18 -mo OS, % Not estimable 79 70 17. 1 60 48 0. 56 (0. 40 -0. 79) --- <. 001 --- Median PFS, † mos § 12 -mo PFS, % 5. 7 36 2. 1 15 0. 56 (0. 43 -0. 73) -- <. 001 -- *Primary endpoint. †By independent radiology review. Powles. ASCO 2020. Abstr LBA 1. Slide credit: clinicaloptions. com
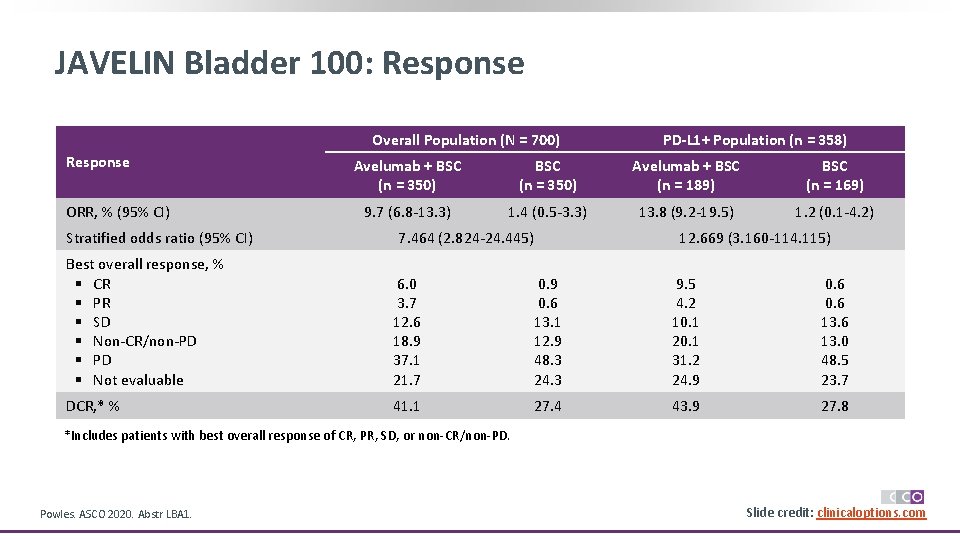
JAVELIN Bladder 100: Response Overall Population (N = 700) Response ORR, % (95% CI) Stratified odds ratio (95% CI) PD-L 1+ Population (n = 358) Avelumab + BSC (n = 350) Avelumab + BSC (n = 189) BSC (n = 169) 9. 7 (6. 8 -13. 3) 1. 4 (0. 5 -3. 3) 13. 8 (9. 2 -19. 5) 1. 2 (0. 1 -4. 2) 7. 464 (2. 824 -24. 445) 12. 669 (3. 160 -114. 115) Best overall response, % § CR § PR § SD § Non-CR/non-PD § Not evaluable 6. 0 3. 7 12. 6 18. 9 37. 1 21. 7 0. 9 0. 6 13. 1 12. 9 48. 3 24. 3 9. 5 4. 2 10. 1 20. 1 31. 2 24. 9 0. 6 13. 0 48. 5 23. 7 DCR, * % 41. 1 27. 4 43. 9 27. 8 *Includes patients with best overall response of CR, PR, SD, or non-CR/non-PD. Powles. ASCO 2020. Abstr LBA 1. Slide credit: clinicaloptions. com
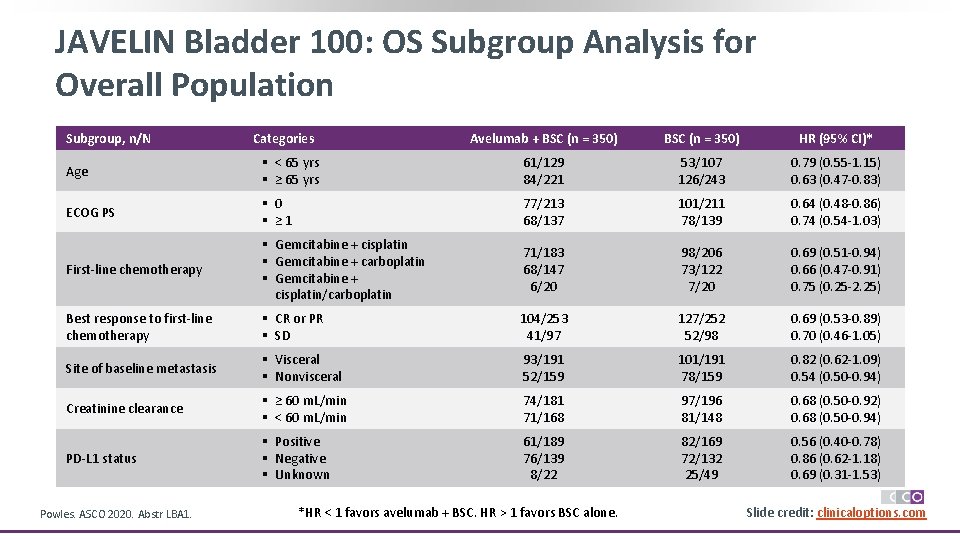
JAVELIN Bladder 100: OS Subgroup Analysis for Overall Population Subgroup, n/N Categories Avelumab + BSC (n = 350) HR (95% CI)* Age § < 65 yrs § ≥ 65 yrs 61/129 84/221 53/107 126/243 0. 79 (0. 55 -1. 15) 0. 63 (0. 47 -0. 83) ECOG PS § 0 § ≥ 1 77/213 68/137 101/211 78/139 0. 64 (0. 48 -0. 86) 0. 74 (0. 54 -1. 03) First-line chemotherapy § Gemcitabine + cisplatin § Gemcitabine + carboplatin § Gemcitabine + cisplatin/carboplatin 71/183 68/147 6/20 98/206 73/122 7/20 0. 69 (0. 51 -0. 94) 0. 66 (0. 47 -0. 91) 0. 75 (0. 25 -2. 25) Best response to first-line chemotherapy § CR or PR § SD 104/253 41/97 127/252 52/98 0. 69 (0. 53 -0. 89) 0. 70 (0. 46 -1. 05) Site of baseline metastasis § Visceral § Nonvisceral 93/191 52/159 101/191 78/159 0. 82 (0. 62 -1. 09) 0. 54 (0. 50 -0. 94) Creatinine clearance § ≥ 60 m. L/min § < 60 m. L/min 74/181 71/168 97/196 81/148 0. 68 (0. 50 -0. 92) 0. 68 (0. 50 -0. 94) PD-L 1 status § Positive § Negative § Unknown 61/189 76/139 8/22 82/169 72/132 25/49 0. 56 (0. 40 -0. 78) 0. 86 (0. 62 -1. 18) 0. 69 (0. 31 -1. 53) Powles. ASCO 2020. Abstr LBA 1. *HR < 1 favors avelumab + BSC. HR > 1 favors BSC alone. Slide credit: clinicaloptions. com
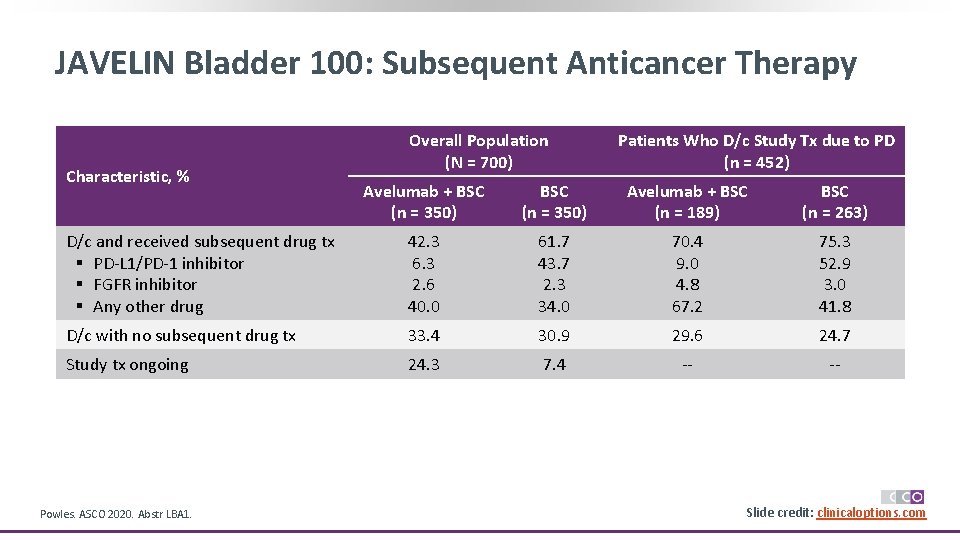
JAVELIN Bladder 100: Subsequent Anticancer Therapy Characteristic, % Overall Population (N = 700) Patients Who D/c Study Tx due to PD (n = 452) Avelumab + BSC (n = 350) Avelumab + BSC (n = 189) BSC (n = 263) D/c and received subsequent drug tx § PD-L 1/PD-1 inhibitor § FGFR inhibitor § Any other drug 42. 3 6. 3 2. 6 40. 0 61. 7 43. 7 2. 3 34. 0 70. 4 9. 0 4. 8 67. 2 75. 3 52. 9 3. 0 41. 8 D/c with no subsequent drug tx 33. 4 30. 9 29. 6 24. 7 Study tx ongoing 24. 3 7. 4 -- -- Powles. ASCO 2020. Abstr LBA 1. Slide credit: clinicaloptions. com
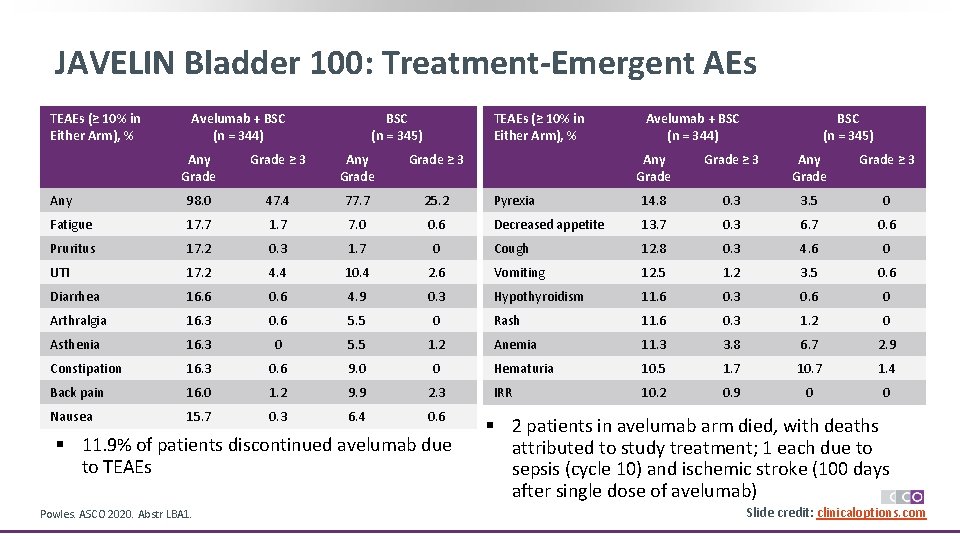
JAVELIN Bladder 100: Treatment-Emergent AEs TEAEs (≥ 10% in Either Arm), % Avelumab + BSC (n = 344) BSC (n = 345) TEAEs (≥ 10% in Either Arm), % Any Grade ≥ 3 Any 98. 0 47. 4 77. 7 25. 2 Fatigue 17. 7 1. 7 7. 0 0. 6 Pruritus 17. 2 0. 3 1. 7 0 UTI 17. 2 4. 4 10. 4 Diarrhea 16. 6 0. 6 Arthralgia 16. 3 Asthenia Avelumab + BSC (n = 344) BSC (n = 345) Any Grade ≥ 3 Pyrexia 14. 8 0. 3 3. 5 0 Decreased appetite 13. 7 0. 3 6. 7 0. 6 Cough 12. 8 0. 3 4. 6 0 2. 6 Vomiting 12. 5 1. 2 3. 5 0. 6 4. 9 0. 3 Hypothyroidism 11. 6 0. 3 0. 6 0 0. 6 5. 5 0 Rash 11. 6 0. 3 1. 2 0 16. 3 0 5. 5 1. 2 Anemia 11. 3 3. 8 6. 7 2. 9 Constipation 16. 3 0. 6 9. 0 0 Hematuria 10. 5 1. 7 10. 7 1. 4 Back pain 16. 0 1. 2 9. 9 2. 3 IRR 10. 2 0. 9 0 0 Nausea 15. 7 0. 3 6. 4 0. 6 § 11. 9% of patients discontinued avelumab due to TEAEs Powles. ASCO 2020. Abstr LBA 1. § 2 patients in avelumab arm died, with deaths attributed to study treatment; 1 each due to sepsis (cycle 10) and ischemic stroke (100 days after single dose of avelumab) Slide credit: clinicaloptions. com
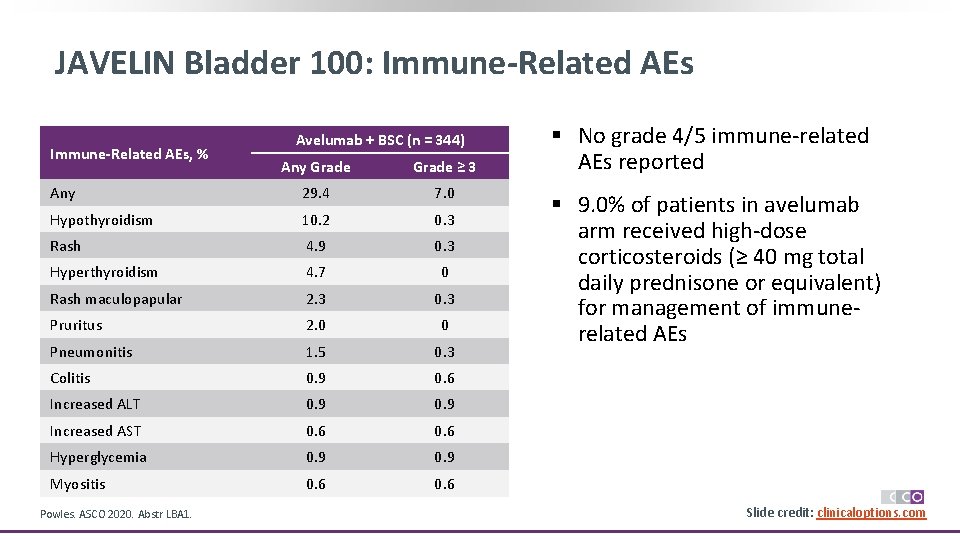
JAVELIN Bladder 100: Immune-Related AEs, % Avelumab + BSC (n = 344) Any Grade ≥ 3 Any 29. 4 7. 0 Hypothyroidism 10. 2 0. 3 Rash 4. 9 0. 3 Hyperthyroidism 4. 7 0 Rash maculopapular 2. 3 0. 3 Pruritus 2. 0 0 Pneumonitis 1. 5 0. 3 Colitis 0. 9 0. 6 Increased ALT 0. 9 Increased AST 0. 6 Hyperglycemia 0. 9 Myositis 0. 6 Powles. ASCO 2020. Abstr LBA 1. § No grade 4/5 immune-related AEs reported § 9. 0% of patients in avelumab arm received high-dose corticosteroids (≥ 40 mg total daily prednisone or equivalent) for management of immunerelated AEs Slide credit: clinicaloptions. com
JAVELIN Bladder 100: Conclusions § JAVELIN Bladder 100 met primary endpoint in overall population and PD-L 1+ population, with maintenance avelumab significantly prolonging OS in patients with response or stable disease after prior first-line platinum chemotherapy ‒ Median OS in overall population: 21. 4 vs 14. 3 mos (HR: 0. 69; 95% CI: 0. 56 -0. 86; P <. 001) ‒ Median OS in PD-L 1+ population: NE vs 17. 1 mos (HR: 0. 56; 95% CI: 0. 40 -0. 79; P <. 001) ‒ OS benefit observed across patient subgroups § Avelumab safety profile deemed manageable, with relatively low rates of grade ≥ 3 AEs; consistent with previous reports § Investigators conclude that avelumab maintenance in patients with advanced urothelial carcinoma whose disease did not progress after first-line, platinum-based chemotherapy represents a new standard-of-care therapy ‒ On June 30, 2020, the FDA approved avelumab as maintenance therapy for patients with locally advanced/metastatic UC that has not progressed on first-line platinum-based chemotherapy Powles. ASCO 2020. Abstr LBA 1. Slide credit: clinicaloptions. com

Go Online for More CCO Coverage of ASCO 2020! Short slideset summaries and additional CME-certified analyses with expert faculty commentary on key studies in: § Breast cancer § Gynecologic cancers § Gastrointestinal cancers § Hematologic malignancies § Genitourinary cancers § Lung cancer clinicaloptions. com/oncology
- Slides: 14