January 2012 Regents Exam http www nysedregents orgchemistry
























- Slides: 35

January 2012 Regents Exam http: //www. nysedregents. org/chemistry/ http: //www. kentchemistry. com/Regents. Exams/regentsexams. htm (very helpful site for chemistry regents practice; Mr. Kent’s chemistry website) http: //newyorkscienceteacher. com/sci/files/user-submitted/200 Ways. Chem. pdf

January 2012 Regents Exam Part C Questions http: //www. nysedregents. org/chemistry/ http: //www. kentchemistry. com/Regents. Exams/regentsexams. htm (very helpful site for chemistry regents practice; Mr. Kent’s chemistry website) http: //newyorkscienceteacher. com/sci/files/user-submitted/200 Ways. Chem. pdf

PRACTICE WITH THE CALCULATOR THAT YOU ARE PERMITTED TO USE FOR THE REGENTS !

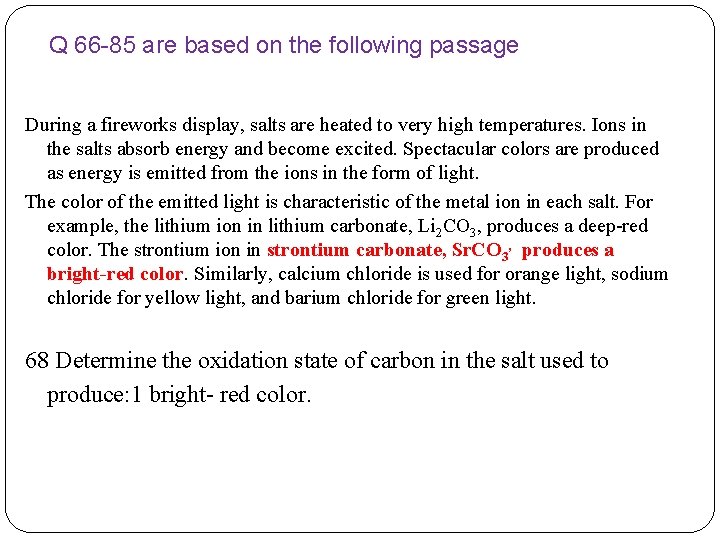
Q 66 -85 are based on the following passage During a fireworks display, salts are heated to very high temperatures. Ions in the salts absorb energy and become excited. Spectacular colors are produced as energy is emitted from the ions in the form of light. The color of the emitted light is characteristic of the metal ion in each salt. For example, the lithium ion in lithium carbonate, Li 2 CO 3, produces a deepred color. The strontium ion in strontium carbonate, Sr. CO 3, produces a bright-red color. Similarly, calcium chloride is used for orange light, sodium chloride for yellow light, and barium chloride for green light. 66 Write the formula for the salt used to produce green light in a fireworks display. 67 Identify the two types of chemical bonds found in the salt used to produce a deep-red color.

Q 66 -85 are based on the following passage During a fireworks display, salts are heated to very high temperatures. Ions in the salts absorb energy and become excited. Spectacular colors are produced as energy is emitted from the ions in the form of light. The color of the emitted light is characteristic of the metal ion in each salt. For example, the lithium ion in lithium carbonate, Li 2 CO 3, produces a deep-red color. The strontium ion in strontium carbonate, Sr. CO 3, produces a bright-red color. Similarly, calcium chloride is used for orange light, sodium chloride for yellow light, and barium chloride for green light. 68 Determine the oxidation state of carbon in the salt used to produce: 1 bright- red color.

Q 66 -85 are based on the following passage During a fireworks display, salts are heated to very high temperatures. Ions in the salts absorb energy and become excited. Spectacular colors are produced as energy is emitted from the ions in the form of light. The color of the emitted light is characteristic of the metal ion in each salt. For example, the lithium ion in lithium carbonate, Li 2 CO 3, produces a deep-red color. The strontium ion in strontium carbonate, Sr. CO 3, produces a bright-red color. Similarly, calcium chloride is used for orange light, sodium chloride for yellow light, and barium chloride for green light. 69 Explain, in terms of subatomic particles and energy states, how the colors in a fireworks display are produced.

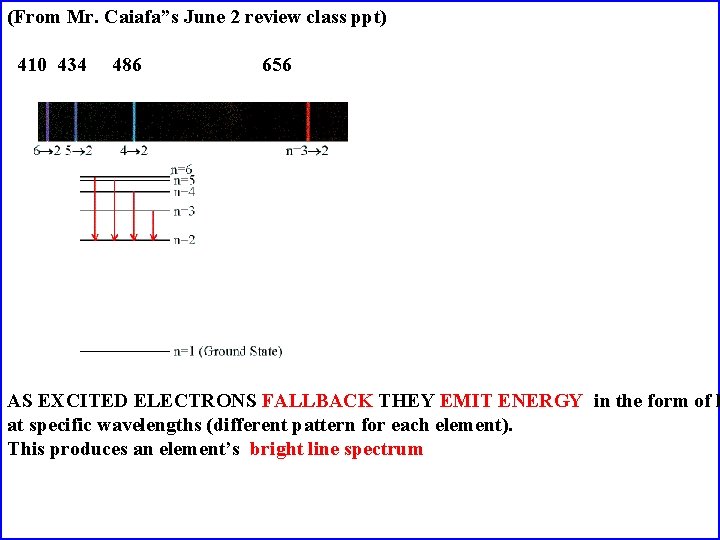
(From Mr. Caiafa”s June 2 review class ppt) 410 434 486 656 AS EXCITED ELECTRONS FALLBACK THEY EMIT ENERGY in the form of l at specific wavelengths (different pattern for each element). This produces an element’s bright line spectrum

Q 66 -85 are based on the following passage During a fireworks display, salts are heated to very high temperatures. Ions in the salts absorb energy and become excited. Spectacular colors are produced as energy is emitted from the ions in the form of light. The color of the emitted light is characteristic of the metal ion in each salt. For example, the lithium ion in lithium carbonate, Li 2 CO 3, produces a deep-red color. The strontium ion in strontium carbonate, Sr. CO 3, produces a bright-red color. Similarly, calcium chloride is used for orange light, sodium chloride for yellow light, and barium chloride for green light. 69 Explain, in terms of subatomic particles and energy states, how the colors in a fireworks display are produced. Possible answer: As electrons fallback from the excited state to lower energy levels they emit energy in the form of light. (different pattern for each element).

Q 66 -85 are based on the following passage During a fireworks display, salts are heated to very high temperatures. Ions in the salts absorb energy and become excited. Spectacular colors are produced as energy is emitted from the ions in the form of light. The color of the emitted light is characteristic of the metal ion in each salt. For example, the lithium ion in lithium carbonate, Li 2 CO 3, produces a deepred color. The strontium ion in strontium carbonate, Sr. CO 3, produces a bright-red color. Similarly, calcium chloride is used for orange light, sodium chloride for yellow light, and barium chloride for green light. 68 Determine the oxidation state of carbon in the salt used to produce: 1 bright- red color.

Q 66 -85 are based on the following passage During a fireworks display, salts are heated to very high temperatures. Ions in the salts absorb energy and become excited. Spectacular colors are produced as energy is emitted from the ions in the form of light. The color of the emitted light is characteristic of the metal ion in each salt. For example, the lithium ion in lithium carbonate, Li 2 CO 3, produces a deep-red color. The strontium ion in strontium carbonate, Sr. CO 3, produces a brightred color. Similarly, calcium chloride is used for orange light, sodium chloride for yellow light, and barium chloride for green light. 66 Write the formula for the salt used to produce green light in a fireworks display.

Base your answers to questions 70 and 71 on the information below. A scientist makes a solution that contains 44. 0 grams of hydrogen chloride gas HCl (g) in 200. 0 grams of water at 20 o. C. The process is represented by the equation below. 70 Based on Reference Table G. identify, in terms of saturation, the type of solution made by the scientist. (see item 107 of 200 ways to pass. . and use solubility tables; pay attention to quantities) 71 Explain, in terms of the distribution of particles, why the solution is a homogeneous mixture. (definition based question)

Base your answers to questions 70 and 71 on the information below. A scientist makes a solution that contains 44. 0 grams of hydrogen chloride gas HCl (g) in 200. 0 grams of water at 20 o. C. The process is represented by the equation below. 70 Based on Reference Table G. identify, in terms of saturation, the type of solution made by the scientist. (see item 107 of 200 ways to pass. . and use solubility tables; pay attention to quantities) Answer = unsaturated 71. Explain, in terms of the distribution of particles, why the solution is a homogeneous mixture. (definition based question) Answer : This is a homogeneous mixture because it has uniform distribution of the ions in solution

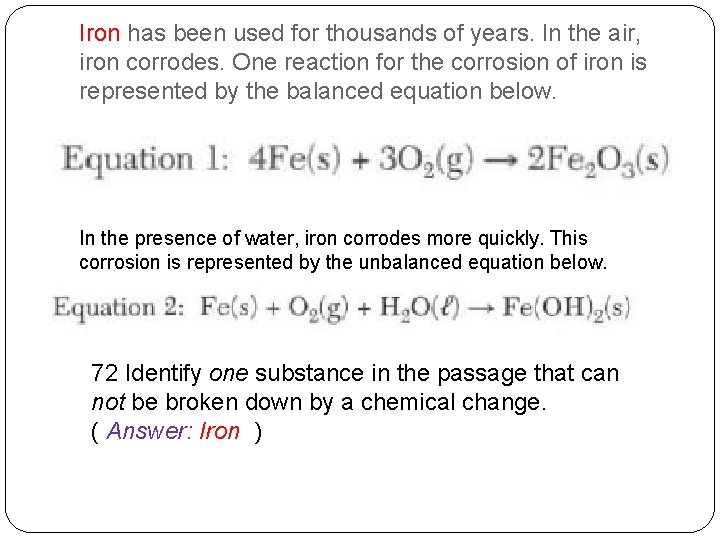
Iron has been used for thousands of years. In the air, iron corrodes. One reaction for the corrosion of iron is represented by the balanced equation below. In the presence of water, iron corrodes more quickly. This corrosion is represented by the unbalanced equation below. 72 Identify one substance in the passage that can not be broken down by a chemical change. ( Q based on definition of an element)

Iron has been used for thousands of years. In the air, iron corrodes. One reaction for the corrosion of iron is represented by the balanced equation below. In the presence of water, iron corrodes more quickly. This corrosion is represented by the unbalanced equation below. 72 Identify one substance in the passage that can not be broken down by a chemical change. ( Answer: Iron )

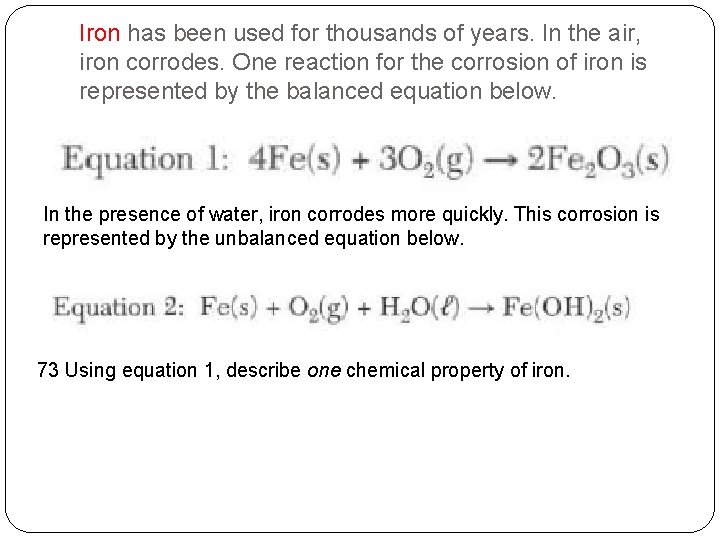
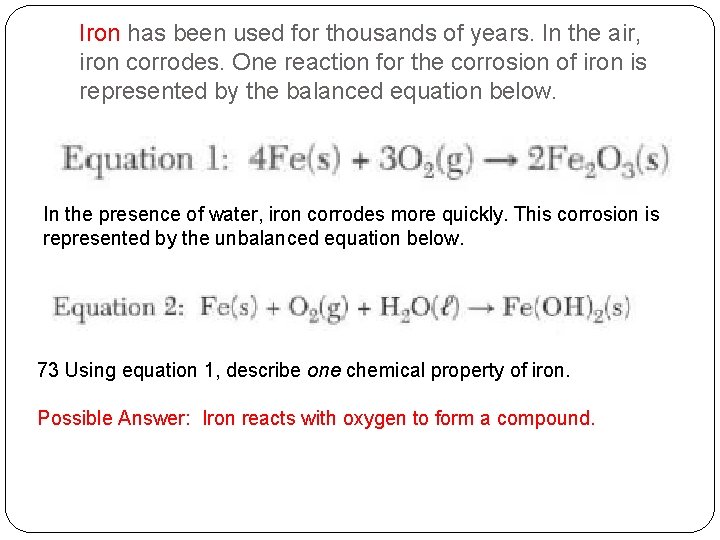
Iron has been used for thousands of years. In the air, iron corrodes. One reaction for the corrosion of iron is represented by the balanced equation below. In the presence of water, iron corrodes more quickly. This corrosion is represented by the unbalanced equation below. 73 Using equation 1, describe one chemical property of iron.

Iron has been used for thousands of years. In the air, iron corrodes. One reaction for the corrosion of iron is represented by the balanced equation below. In the presence of water, iron corrodes more quickly. This corrosion is represented by the unbalanced equation below. 73 Using equation 1, describe one chemical property of iron. Possible Answer: Iron reacts with oxygen to form a compound.

Iron has been used for thousands of years. In the air, iron corrodes. One reaction for the corrosion of iron is represented by the balanced equation below. In the presence of water, iron corrodes more quickly. This corrosion is represented by the unbalanced equation below. 74 Balance the equation in your answer booklet, using the smallest whole-number coefficients. ___ Fe(s) + __ O 2 (g) + ___ H 2 O (l) ___Fe(OH) 2 (s)

Iron has been used for thousands of years. In the air, iron corrodes. One reaction for the corrosion of iron is represented by the balanced equation below. In the presence of water, iron corrodes more quickly. This corrosion is represented by the unbalanced equation below. 74 Balance the equation in your answer booklet, using the smallest whole-number coefficients. 2 Fe(s) + O 2 (g) + 2 H 2 O (l) 2 Fe(OH) 2 (s)

Vitamin C, also known as ascorbic acid, is water soluble and cannot be produced by the human body. Each day, a person's diet should include a source of vitamin C, such as orange juice. Ascorbic acid has a molecular formula of C 6 H 8 O 6 and a gram-formula mass of 176 grams per mole. 75 What is the color of the indicator thymol blue after it is added to an aqueous solution of vitamin C? (Hint: Use Indicator table) 76 Determine the number of moles of vitamin C in an orange that contains 0. 071 gram of vitamin C.

Vitamin C, also known as ascorbic acid, is water soluble and cannot be produced by the human body. Each day, a person's diet should include a source of vitamin C, such as orange juice. Ascorbic acid has a molecular formula of C 6 H 8 O 6 and a gram-formula mass of 176 grams per mole. 75 What is the color of the indicator thymol blue after it is added to an aqueous solution of vitamin C? (Hint: Use Indicator table) 76 Determine the number of moles of vitamin C in an orange that contains 0. 071 gram of vitamin C. (You will need to use the GFM)

Vitamin C, also known as ascorbic acid, is water soluble and cannot be produced by the human body. Each day, a person's diet should include a source of vitamin C, such as orange juice. Ascorbic acid has a molecular formula of C 6 H 8 O 6 and a gram-formula mass of 176 grams per mole. 77 In the space in your answer booklet, show a numerical setup for calculating the percent composition by mass of oxygen in ascorbic acid. 78 Write the empirical formula for ascorbic acid.

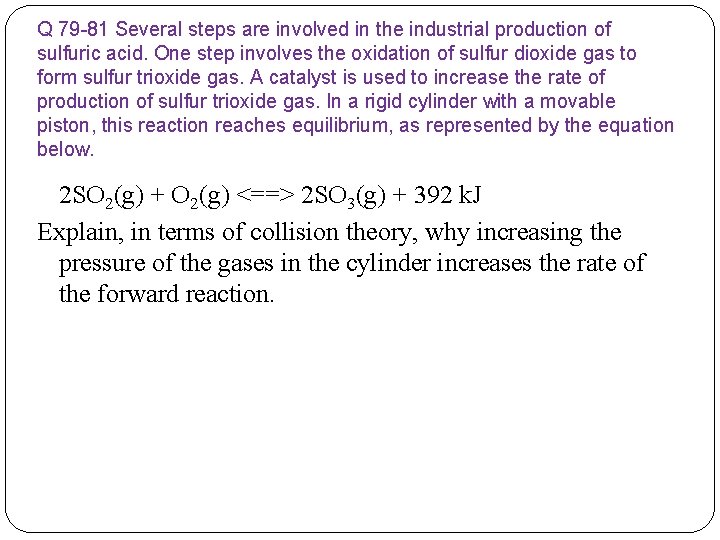
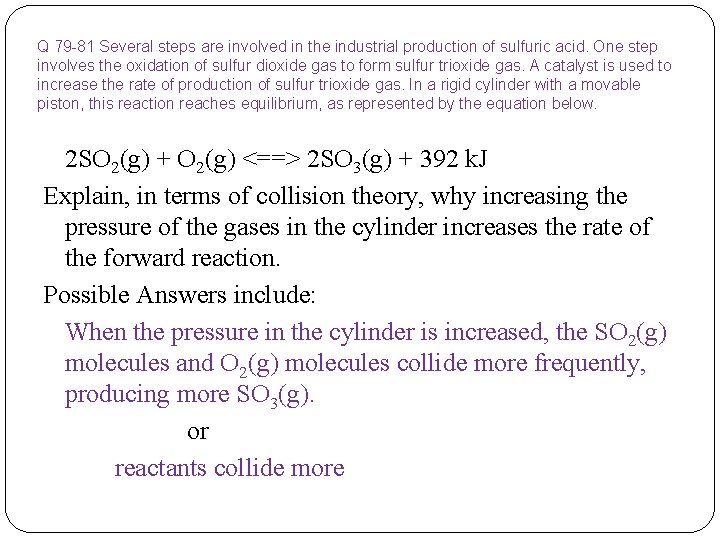
Q 79 -81 Several steps are involved in the industrial production of sulfuric acid. One step involves the oxidation of sulfur dioxide gas to form sulfur trioxide gas. A catalyst is used to increase the rate of production of sulfur trioxide gas. In a rigid cylinder with a movable piston, this reaction reaches equilibrium, as represented by the equation below. 2 SO 2(g) + O 2(g) <==> 2 SO 3(g) + 392 k. J Explain, in terms of collision theory, why increasing the pressure of the gases in the cylinder increases the rate of the forward reaction.

Q 79 -81 Several steps are involved in the industrial production of sulfuric acid. One step involves the oxidation of sulfur dioxide gas to form sulfur trioxide gas. A catalyst is used to increase the rate of production of sulfur trioxide gas. In a rigid cylinder with a movable piston, this reaction reaches equilibrium, as represented by the equation below. 2 SO 2(g) + O 2(g) <==> 2 SO 3(g) + 392 k. J Explain, in terms of collision theory, why increasing the pressure of the gases in the cylinder increases the rate of the forward reaction. Possible Answers include: When the pressure in the cylinder is increased, the SO 2(g) molecules and O 2(g) molecules collide more frequently, producing more SO 3(g). or reactants collide more

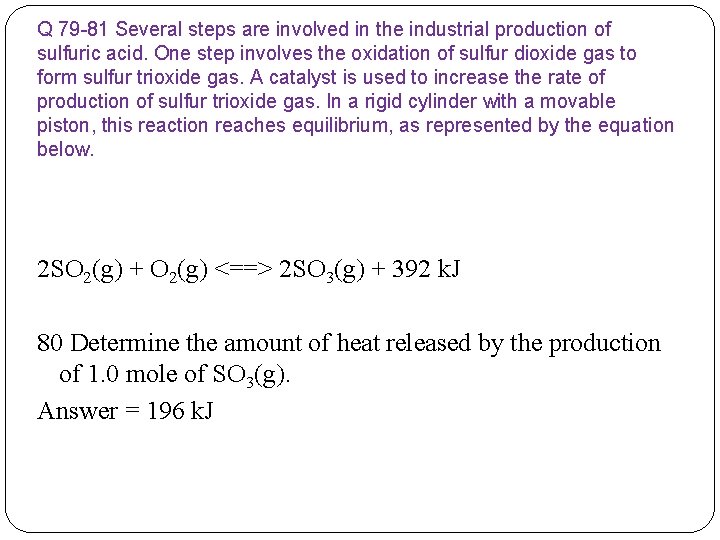
Q 79 -81 Several steps are involved in the industrial production of sulfuric acid. One step involves the oxidation of sulfur dioxide gas to form sulfur trioxide gas. A catalyst is used to increase the rate of production of sulfur trioxide gas. In a rigid cylinder with a movable piston, this reaction reaches equilibrium, as represented by the equation below. 2 SO 2(g) + O 2(g) <==> 2 SO 3(g) + 392 k. J 80 Determine the amount of heat released by the production of 1. 0 mole of SO 3(g).

Q 79 -81 Several steps are involved in the industrial production of sulfuric acid. One step involves the oxidation of sulfur dioxide gas to form sulfur trioxide gas. A catalyst is used to increase the rate of production of sulfur trioxide gas. In a rigid cylinder with a movable piston, this reaction reaches equilibrium, as represented by the equation below. 2 SO 2(g) + O 2(g) <==> 2 SO 3(g) + 392 k. J 80 Determine the amount of heat released by the production of 1. 0 mole of SO 3(g). Answer = 196 k. J

Q 79 -81 Several steps are involved in the industrial production of sulfuric acid. One step involves the oxidation of sulfur dioxide gas to form sulfur trioxide gas. A catalyst is used to increase the rate of production of sulfur trioxide gas. In a rigid cylinder with a movable piston, this reaction reaches equilibrium, as represented by the equation below. 2 SO 2(g) + O 2(g) <==> 2 SO 3(g) + 392 k. J 81 State, in terms of the concentration of SO 3(g) , what occurs when more O 2(g) is added to the reaction at equilibrium.

Q 79 -81 Several steps are involved in the industrial production of sulfuric acid. One step involves the oxidation of sulfur dioxide gas to form sulfur trioxide gas. A catalyst is used to increase the rate of production of sulfur trioxide gas. In a rigid cylinder with a movable piston, this reaction reaches equilibrium, as represented by the equation below. 2 SO 2(g) + O 2(g) <==> 2 SO 3(g) + 392 k. J 81 State, in terms of the concentration of SO 3(g) , what occurs when more O 2(g) is added to the reaction at equilibrium. Answer: The concentration of SO 3 will increase

For Q 82 -85 see Items 177 -189 of 200 ways to pass…. Base your answers to questions 82 through 85 on the information below. Nuclear radiation is harmful to living cells, particularly to fastgrowing cells, such as cancer cells and blood cells. An external beam of the radiation emitted from a radioisotope can be directed on a small area of a person to destroy cancer cells within the body. Cobalt-60 is an artificially produced radioisotope that emits gamma rays and beta particles. One hospital keeps a 100. 0 gram sample of cobalt-60 in an appropriate, secure storage container for future cancer treatment. 82 State one risk to human tissue associated with the use of radioisotopes to treat cancer.

For Q 82 -85 see Items 177 -189 of 200 ways to pass…. Base your answers to questions 82 through 85 on the information below. Nuclear radiation is harmful to living cells, particularly to fastgrowing cells, such as cancer cells and blood cells. An external beam of the radiation emitted from a radioisotope can be directed on a small area of a person to destroy cancer cells within the body. Cobalt-60 is an artificially produced radioisotope that emits gamma rays and beta particles. One hospital keeps a 100. 0 gram sample of cobalt-60 in an appropriate, secure storage container for future cancer treatment. 82 State one risk to human tissue associated with the use of radioisotopes to treat cancer. The radiation is harmful to fast growing cells such as blood

Base your answers to questions 82 through 85 on the information below. Nuclear radiation is harmful to living cells, particularly to fast-growing cells, such as cancer cells and blood cells. An external beam of the radiation emitted from a radioisotope can be directed on a small area of a person to destroy cancer cells within the body. Cobalt-60 is an artificially produced radioisotope that emits gamma rays and beta particles. One hospital keeps a 100. 0 -gram sample of cobalt-60 in an appropriate, secure storage container for future cancer treatment. 83 Compare the penetrating power of the two emissions from the Co-60.

Base your answers to questions 82 through 85 on the information below. Nuclear radiation is harmful to living cells, particularly to fast-growing cells, such as cancer cells and blood cells. An external beam of the radiation emitted from a radioisotope can be directed on a small area of a person to destroy cancer cells within the body. Cobalt-60 is an artificially produced radioisotope that emits gamma rays and beta particles. One hospital keeps a 100. 0 -gram sample of cobalt-60 in an appropriate, secure storage container for future cancer treatment. 84 Complete the nuclear equation in your answer booklet for the beta decay of the Co-60 by writing an isotopic notation for the missing product.

Base your answers to questions 82 through 85 on the information below. Nuclear radiation is harmful to living cells, particularly to fast-growing cells, such as cancer cells and blood cells. An external beam of the radiation emitted from a radioisotope can be directed on a small area of a person to destroy cancer cells within the body. Cobalt-60 is an artificially produced radioisotope that emits gamma rays and beta particles. One hospital keeps a 100. 0 -gram sample of cobalt-60 in an appropriate, secure storage container for future cancer treatment. 85 Determine the total time that will have elapsed when 12. 5 grams of the original Co-60 sample at the hospital remains unchanged. Ø Original mass = 100 g Ø Final mass = 12. 5 g Ø Number of half lives that have passed =? Total time elapsed=? Ø Half-life of Co-60 = ? (Use Table N; Co-60 half-life = 5. 271 y)

Base your answers to questions 82 through 85 on the information below. Nuclear radiation is harmful to living cells, particularly to fast-growing cells, such as cancer cells and blood cells. An external beam of the radiation emitted from a radioisotope can be directed on a small area of a person to destroy cancer cells within the body. Cobalt-60 is an artificially produced radioisotope that emits gamma rays and beta particles. One hospital keeps a 100. 0 -gram sample of cobalt-60 in an appropriate, secure storage container for future cancer treatment. 85 Determine the total time that will have elapsed when 12. 5 grams of the original Co-60 sample at the hospital remains unchanged. Ø Original mass = 100 g Ø Final mass = 12. 5 g Ø Number of half lives that have passed =? Total time elapsed=? Ø Half-life of Co-60 = ? (Use Table N; Co-60 half-life = 5. 271 y)

Determine the total time that will have elapsed when 12. 5 grams of the original 100 gram Co-60 sample at the hospital remains unchanged. Steps: (1) Setup figure to determine how many half-lives it took for 100 g to decay to 12. 5 (2) Use the half-life and the number of half-lives to determine the total elapsed time by using the following relationship # of half-lives = total elapsed time ÷ half-life of isotope 3 half-lives= x years ÷ (5. 271 y )

Determine the total time that will have elapsed when 12. 5 grams of the original 100 gram Co-60 sample at the hospital remains unchanged. Steps: (1) Setup figure to determine how many half-lives it took for 100 g to decay to 12. 5 (2) Use the half-life and the number of half-lives to determine the total elapsed time by using the following relationship # of half-lives = total elapsed time ÷ half-life of isotope 3 half-lives= x years ÷ (5. 271 ) Elapsed time = 5. 271 y x 3 = 15. 8 y 100 g 50 g 25 g 12. 5 g
 January 2018 chemistry regents answers
January 2018 chemistry regents answers Nysedregents
Nysedregents Nysedregents chemistry
Nysedregents chemistry January 2012
January 2012 Http //mbs.meb.gov.tr/ http //www.alantercihleri.com
Http //mbs.meb.gov.tr/ http //www.alantercihleri.com Http //siat.ung.ac.id atau http //pmb.ung.ac.id
Http //siat.ung.ac.id atau http //pmb.ung.ac.id Regents chemistry bonding review sheet
Regents chemistry bonding review sheet Crq global regents
Crq global regents Tristan denley
Tristan denley American history regents review
American history regents review Earth science regents lab practical
Earth science regents lab practical Alviz biz resources
Alviz biz resources Regents professor emeritus
Regents professor emeritus Physics regents
Physics regents Comprehensive english regents
Comprehensive english regents Global 9 regents
Global 9 regents Table f chemistry reference table
Table f chemistry reference table June 2010 physics regents answers
June 2010 physics regents answers Which title best completes the graphic organizer?
Which title best completes the graphic organizer? Us history regents review
Us history regents review Moles conversion chart
Moles conversion chart Rocks cluster tutorial
Rocks cluster tutorial Evison regents
Evison regents How to start an enduring issues essay
How to start an enduring issues essay Regents biology food chains and energy in ecosystems
Regents biology food chains and energy in ecosystems Beaks of finches lab answers
Beaks of finches lab answers Thematic essay human rights
Thematic essay human rights June 2010 chemistry regents answers
June 2010 chemistry regents answers Colligative properties regents questions
Colligative properties regents questions Regents of university of california v bakke
Regents of university of california v bakke La board of regents
La board of regents Ccela
Ccela Earth science lab practical
Earth science lab practical United states history and government regents
United states history and government regents Which map shows the most probable areas of precipitation
Which map shows the most probable areas of precipitation June 2019 geometry regents answers
June 2019 geometry regents answers