Its All Elementary Chemistry Basics The teeny tiny

It’s All Elementary: Chemistry Basics
The teeny, tiny stuff: Atom: Smallest particle of an element that retains the characteristics of that element. Element: The pure chemical substance containing only one type of atom Compound: A combination of elements, chemically bonded
Essential Elements o An element is a pure substance that contains only one type of atom. n Examples: H – hydrogen C – carbon O – oxygen o Elements are usually found combined with other elements as compounds. n Compound – a substance formed when two or more elements are chemically bonded. o Example – C 6 H 12 O 6 - Sugar
Structure of an atom: o The basic unit of matter is the atom. o Atoms have three basic parts: n Protons (+ charge) n Neutrons (no charge) n Electrons (- charge) o These are the tiniest and weigh practically nothing.
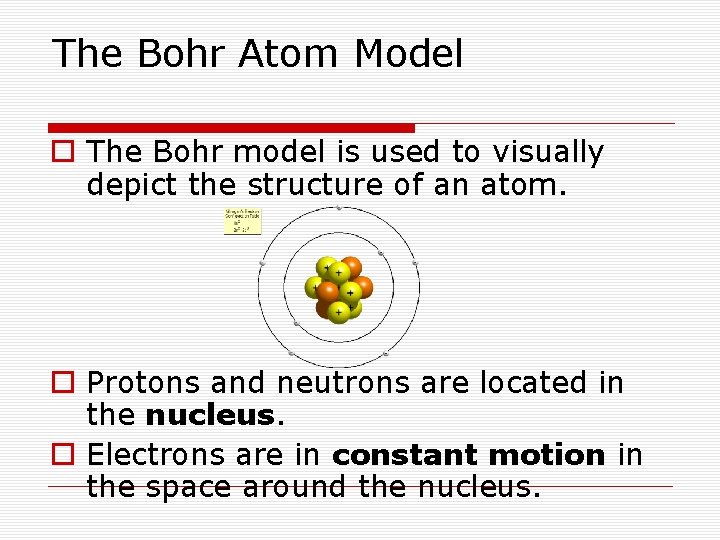
The Bohr Atom Model o The Bohr model is used to visually depict the structure of an atom. o Protons and neutrons are located in the nucleus. o Electrons are in constant motion in the space around the nucleus.
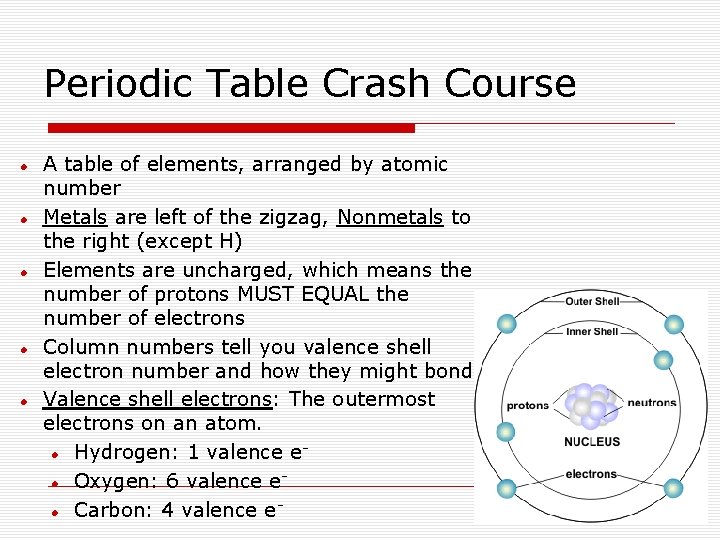
Periodic Table Crash Course A table of elements, arranged by atomic number Metals are left of the zigzag, Nonmetals to the right (except H) Elements are uncharged, which means the number of protons MUST EQUAL the number of electrons Column numbers tell you valence shell electron number and how they might bond Valence shell electrons: The outermost electrons on an atom. Hydrogen: 1 valence e Oxygen: 6 valence e Carbon: 4 valence e-
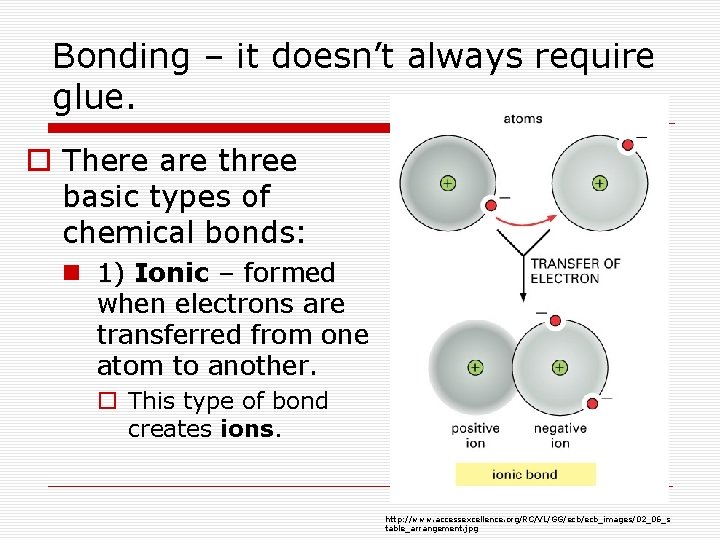
Bonding – it doesn’t always require glue. o There are three basic types of chemical bonds: n 1) Ionic – formed when electrons are transferred from one atom to another. o This type of bond creates ions. http: //www. accessexcellence. org/RC/VL/GG/ecb_images/02_06_s table_arrangement. jpg
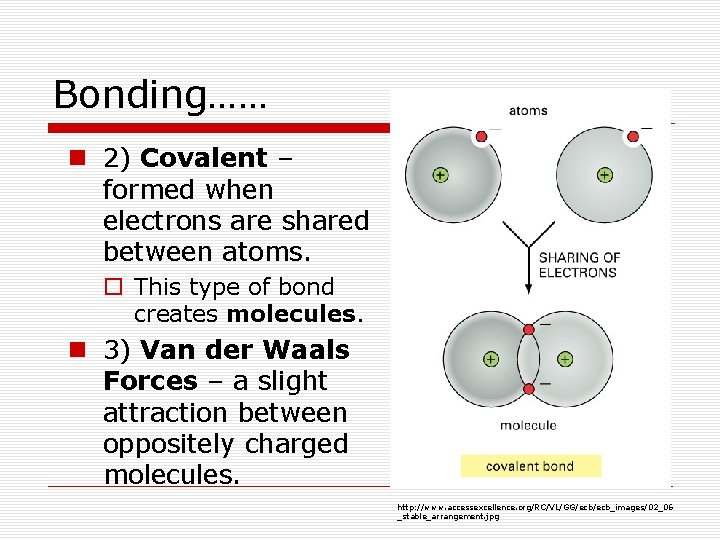
Bonding…… n 2) Covalent – formed when electrons are shared between atoms. o This type of bond creates molecules. n 3) Van der Waals Forces – a slight attraction between oppositely charged molecules. http: //www. accessexcellence. org/RC/VL/GG/ecb_images/02_06 _stable_arrangement. jpg

One more bond type…Van der Waals Forces When molecules are close together, a slight attraction can develop between the oppositely charged regions of nearby molecules. Copyright Pearson Prentice Hall
Demo of covalent bonding o http: //www. teachersd omain. org/asset/lsps 0 7_int_covalentbond/ http: //openlearn. open. ac. uk/file. php/1605/SK 1 83_1_002 i. jpg
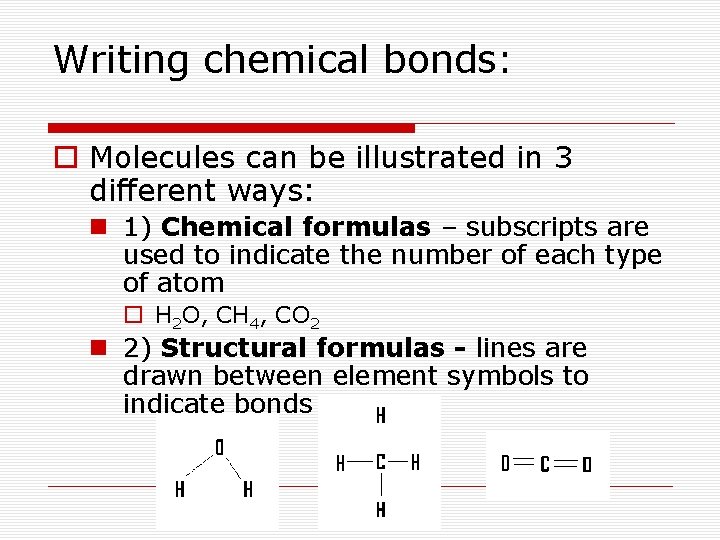
Writing chemical bonds: o Molecules can be illustrated in 3 different ways: n 1) Chemical formulas – subscripts are used to indicate the number of each type of atom o H 2 O, CH 4, CO 2 n 2) Structural formulas - lines are drawn between element symbols to indicate bonds
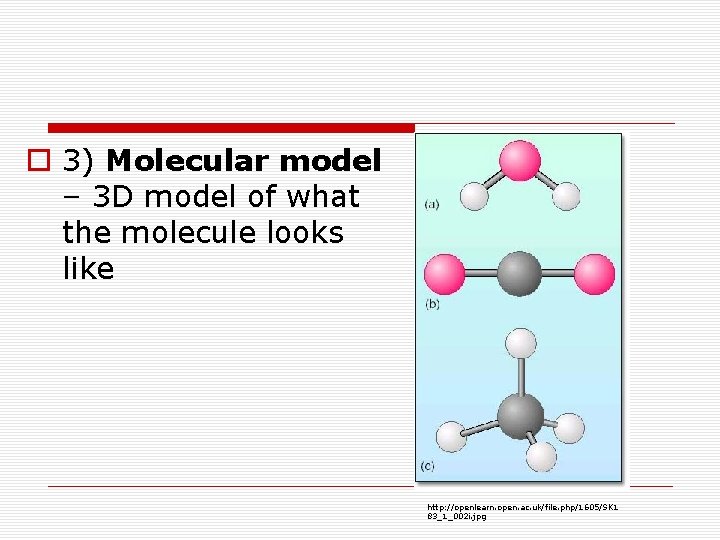
o 3) Molecular model – 3 D model of what the molecule looks like http: //openlearn. open. ac. uk/file. php/1605/SK 1 83_1_002 i. jpg

Compounds are NOT Mixtures Mixture: A combination of substances where both keep their original properties. Solution: One or more substances (solutes) are distributed evenly in another substance (solvent) Physically mixed, not chemically mixed. The more solute in a solvent, the higher the concentration. Suspensions: Mixtures of water and non dissolved particles Example: Blood
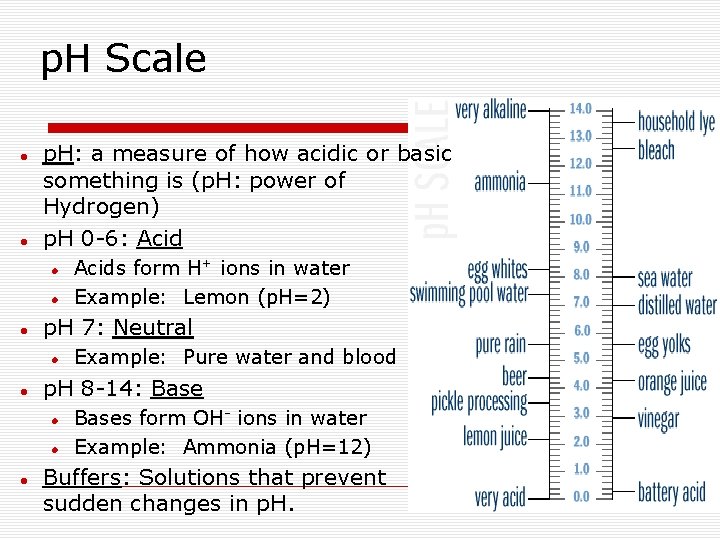
p. H Scale p. H: a measure of how acidic or basic something is (p. H: power of Hydrogen) p. H 0 -6: Acid p. H 7: Neutral Example: Pure water and blood p. H 8 -14: Base Acids form H+ ions in water Example: Lemon (p. H=2) Bases form OH- ions in water Example: Ammonia (p. H=12) Buffers: Solutions that prevent sudden changes in p. H.
- Slides: 14