Istituto Nazionale per le malattie Infettive L Spallanzani



































- Slides: 68


Istituto Nazionale per le malattie Infettive L. Spallanzani Roma, Italy Immunodiagnosis of TB Delia Goletti Borstel, May 28 th, 2010

Agenda n Problems in the diagnosis of TB n TST n IGRA n n Quanti. FERON-TB RD 1 -based assays: n n n T-SPOT TB Quanti. FERON-TB Gold New experimental tests

Agenda n Problems in the diagnosis of TB n TST n IGRA n n Quanti. FERON-TB RD 1 -based assays: n n n T-SPOT TB Quanti. FERON-TB Gold New experimental tests

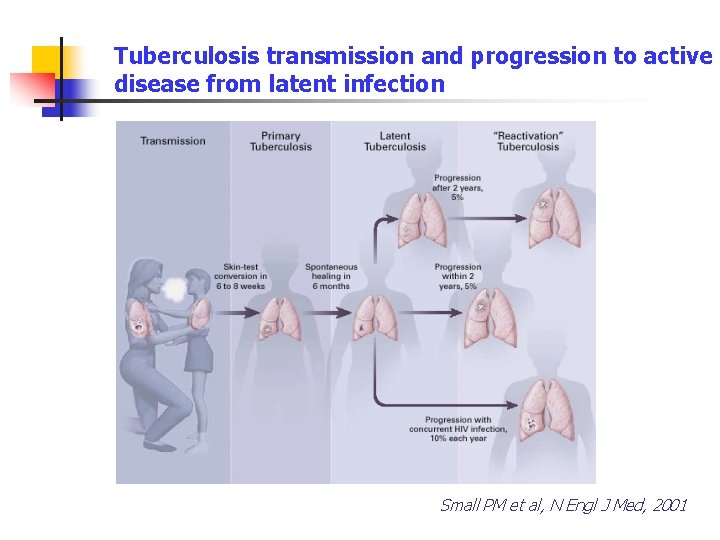
Tuberculosis transmission and progression to active disease from latent infection Small PM et al, N Engl J Med, 2001

The challenge of detecting M. tuberculosis infection n Active disease n n Often difficult to isolate: even with good microbiological facilities, the bacillus is recovered in only 60% of cases Latent infection n M. tuberculosis cannot be cultured from latently infected individuals: no gold standard

Agenda n Problems in the diagnosis of TB n TST n IGRA n n Quanti. FERON-TB RD 1 -based assays: n n n T-SPOT TB Quanti. FERON-TB Gold New experimental tests

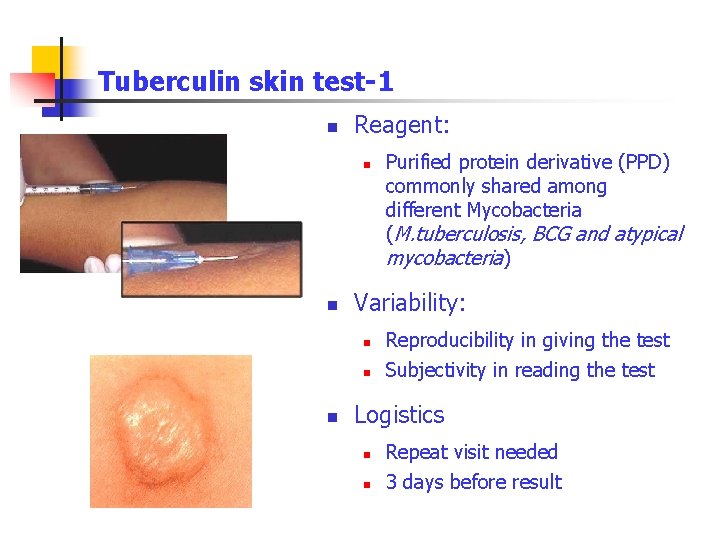
Tuberculin skin test-1 n Reagent: n n Variability: n n n Purified protein derivative (PPD) commonly shared among different Mycobacteria (M. tuberculosis, BCG and atypical mycobacteria) Reproducibility in giving the test Subjectivity in reading the test Logistics n n Repeat visit needed 3 days before result

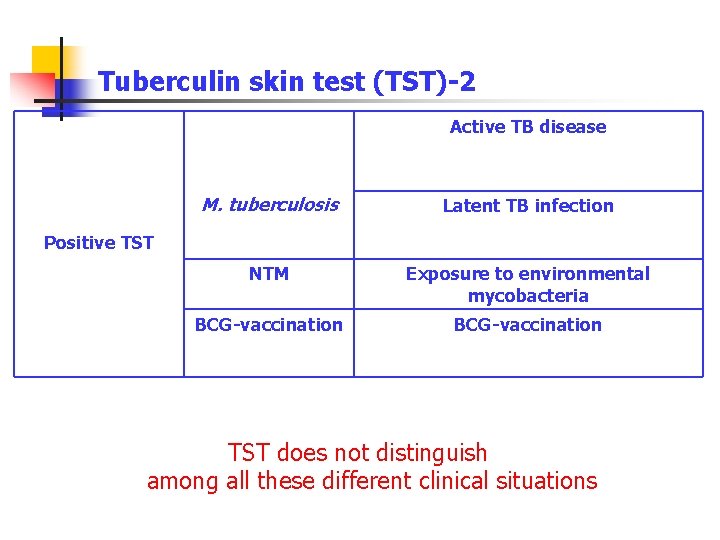
Tuberculin skin test (TST)-2 Active TB disease M. tuberculosis Latent TB infection NTM Exposure to environmental mycobacteria BCG-vaccination Positive TST does not distinguish among all these different clinical situations

Tuberculin skin test-3 n False Negatives n skin reaction is a very crude measure: n n Small responses not picked up (real problem in immunosuppressed patients) 10 -25% negative results in active disease (only 75 -90% sensitivity, worse in immunosuppressed)

Need of… n Standardized test (laboratory test) n M. tuberculosis-specific reagents n Possibility to discriminate between the different stages of tuberculosis

Need of… n Standardized test (laboratory test) n M. tuberculosis-specific reagents n Possibility to discriminate between the different stages of tuberculosis

Agenda n Problems in the diagnosis of TB n TST n IGRA n n Quanti. FERON-TB RD 1 -based assays: n n n T-SPOT TB Quanti. FERON-TB Gold New experimental tests

Quanti. FERON®-TB n Nil n Tuberculin PPD n M. Avium PPD n Mitogen

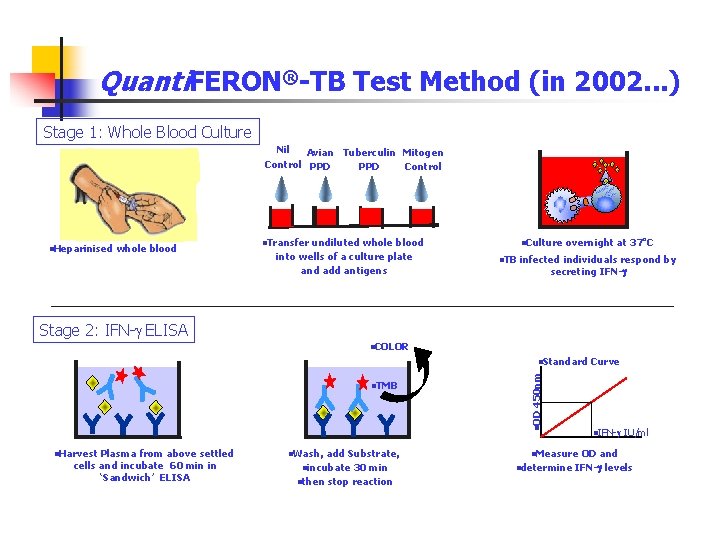
Quanti. FERON®-TB Test Method (in 2002. . . ) Stage 1: Whole Blood Culture Nil Avian Tuberculin Mitogen Control PPD Control n. Heparinised whole blood n. Transfer undiluted whole blood into wells of a culture plate and add antigens n. Culture n. TB overnight at 37 o. C infected individuals respond by secreting IFN-g Stage 2: IFN-g ELISA n. COLOR n. OD n. TMB n. Harvest Plasma from above settled cells and incubate 60 min in ‘Sandwich’ ELISA n. Wash, add Substrate, 30 min nthen stop reaction nincubate Curve 450 nm n. Standard n. IFN-g IU/ml n. Measure ndetermine OD and IFN-g levels

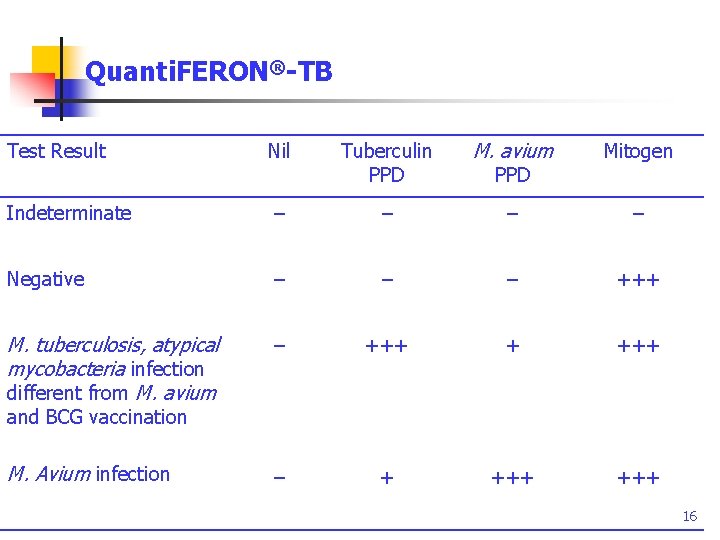
Quanti. FERON®-TB Test Result Nil Tuberculin PPD M. avium Mitogen Indeterminate – – Negative – – – +++ M. tuberculosis, atypical mycobacteria infection different from M. avium – +++ + +++ M. Avium infection – + +++ PPD and BCG vaccination 16

Need of… n Standardized test (laboratory test) n M. tuberculosis-specific reagents n Possibility to discriminate between the different stages of tuberculosis

Agenda n Problems in the diagnosis of TB n TST n IGRA n n Quanti. FERON-TB RD 1 -based assays: n n n T-SPOT TB Quanti. FERON-TB Gold New experimental tests

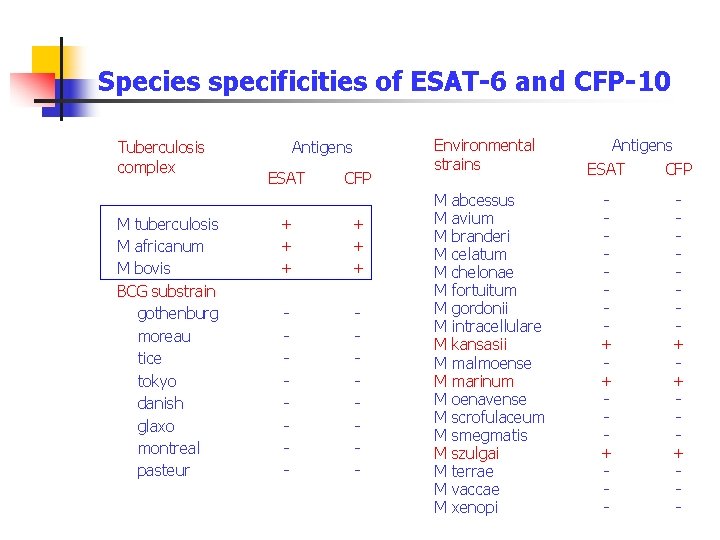
Species specificities of ESAT-6 and CFP-10 Tuberculosis complex M tuberculosis M africanum M bovis BCG substrain gothenburg moreau tice tokyo danish glaxo montreal pasteur Antigens ESAT CFP + + + - - Environmental strains Antigens ESAT CFP M abcessus M avium M branderi M celatum M chelonae M fortuitum M gordonii M intracellulare M kansasii M malmoense M marinum M oenavense M scrofulaceum M smegmatis M szulgai M terrae M vaccae M xenopi + + + -

Agenda n Problems in the diagnosis of TB n TST n Quanti. FERON-TB n RD 1 -based assays: n n n T-SPOT TB Quanti. FERON-TB Gold New experimental tests

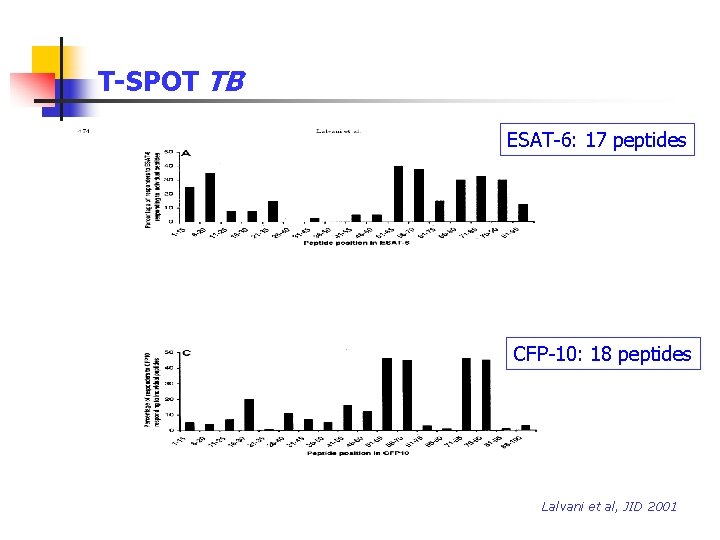
T-SPOT TB ESAT-6: 17 peptides CFP-10: 18 peptides Lalvani et al, JID 2001

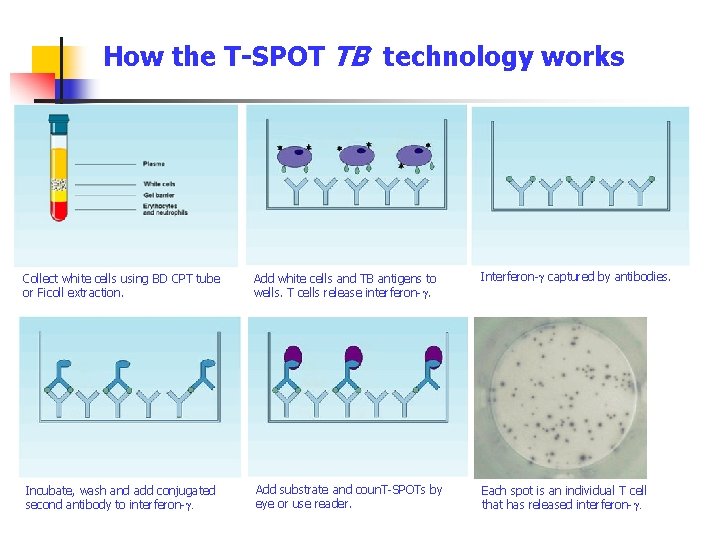
How the T-SPOT TB technology works Collect white cells using BD CPT tube or Ficoll extraction. Add white cells and TB antigens to wells. T cells release interferon-g. Interferon-g captured by antibodies. Incubate, wash and add conjugated second antibody to interferon-g. Add substrate and coun. T-SPOTs by eye or use reader. Each spot is an individual T cell that has released interferon-g.

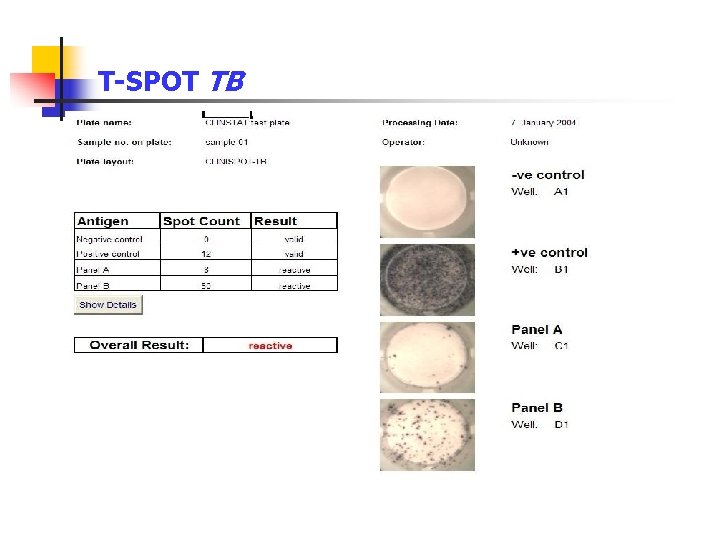
T-SPOT TB

T-SPOT TB n T-SPOT TB™ is a patented method to detect pathogen-specific T cells. n n n A simplified variant of the ex vivo elispot method developed by Dr. Ajit Lalvani Complete system - kit + instrumentation Validated and produced to international quality standards (ISO 13485: 2003, GMP) n Standardized n Quality-controlled n CE marked for in vitro diagnostic use

Agenda n Problems in the diagnosis of TB n TST n IGRA n n Quanti. FERON-TB RD 1 -based assays: n n n T-SPOT TB Quanti. FERON-TB Gold New experimental tests

Quanti. FERON®-TB Gold In tube: peptides used CFP-10: 6 peptides ESAT-6: 7 peptides Mori et al, AJRCCM 2004 TB 7. 7: 1 peptide

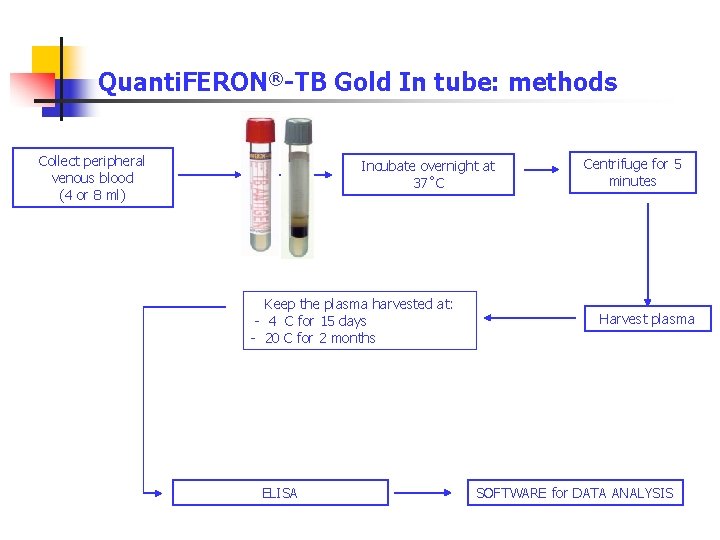
Quanti. FERON®-TB Gold In tube: methods Collect peripheral venous blood (4 or 8 ml) Incubate overnight at 37˚C Keep the plasma harvested at: - 4 C for 15 days - 20 C for 2 months ELISA Centrifuge for 5 minutes Harvest plasma SOFTWARE for DATA ANALYSIS

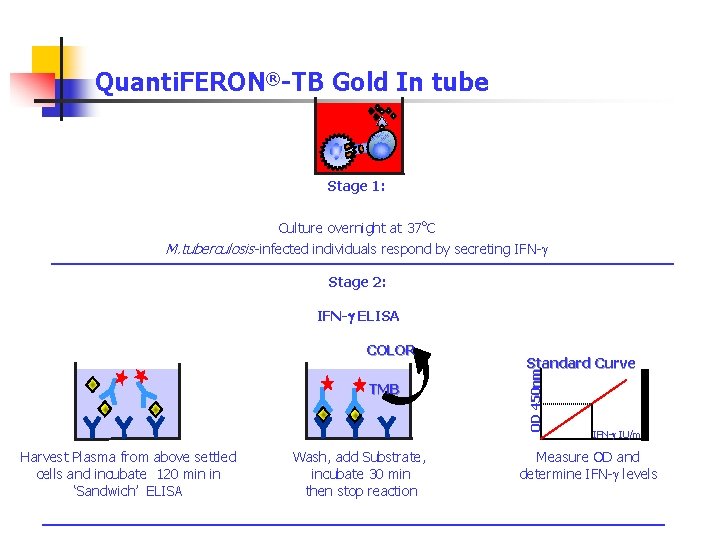
Quanti. FERON®-TB Gold In tube Stage 1: Culture overnight at 37 o. C M. tuberculosis-infected individuals respond by secreting IFN-g Stage 2: IFN-g ELISA TMB Harvest Plasma from above settled cells and incubate 120 min in ‘Sandwich’ ELISA Wash, add Substrate, incubate 30 min then stop reaction Standard Curve OD 450 nm COLOR IFN-g IU/ml Measure OD and determine IFN-g levels

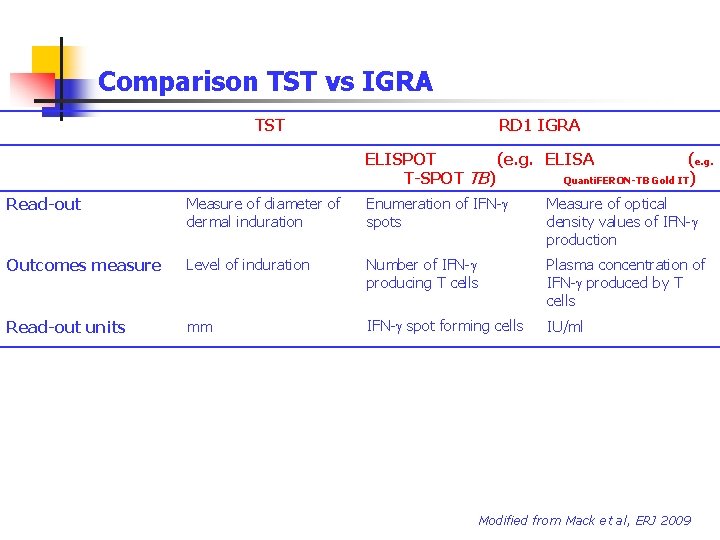
Comparison TST vs IGRA TST RD 1 IGRA ELISPOT (e. g. ELISA (e. g. T-SPOT TB) Quanti. FERON-TB Gold IT) Antigens PPD Peptides from CFP-10, ESAT-6 and TB 7. 7 Tests’ substrate Skin PBMC Whole Blood Time required for the results 72 h 24 h Cells involved Cytokines involved Neutrophils, CD 4, CD 8 that transmigrate out of capillaries into the skin. Treg (CD 4+CD 25 high. Fox. P 3+). IFN-g, TNF-a, TNF-b CD 4 T cells in vitro IFN-g Modified from Mack et al, ERJ 2009

Comparison TST vs IGRA TST RD 1 IGRA ELISPOT (e. g. ELISA (e. g. T-SPOT TB) Quanti. FERON-TB Gold IT) Read-out Measure of diameter of dermal induration Enumeration of IFN-g spots Measure of optical density values of IFN-g production Outcomes measure Level of induration Number of IFN-g producing T cells Plasma concentration of IFN-g produced by T cells Read-out units mm IFN-g spot forming cells IU/ml Modified from Mack et al, ERJ 2009

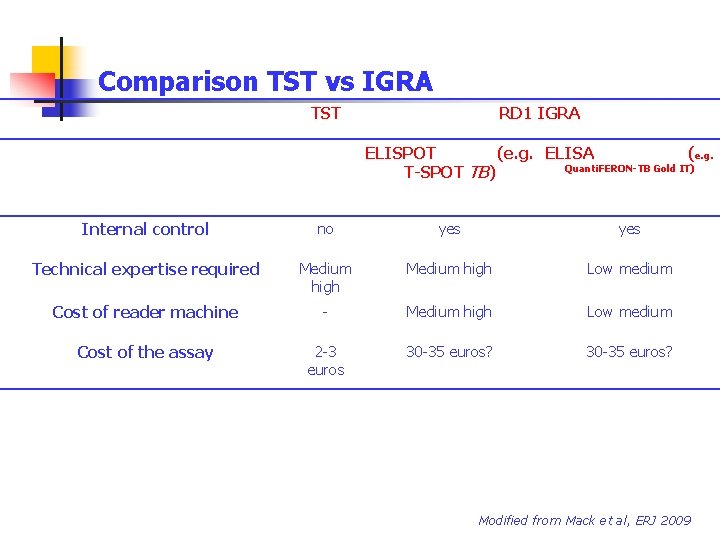
Comparison TST vs IGRA TST RD 1 IGRA ELISPOT (e. g. ELISA (e. g. Quanti. FERON-TB Gold IT) T-SPOT TB) Internal control no yes Technical expertise required Medium high Low medium Cost of reader machine - Medium high Low medium Cost of the assay 2 -3 euros 30 -35 euros? Modified from Mack et al, ERJ 2009

Accuracy n TST n T-SPOT TB n Quanti. FERON-TB Gold

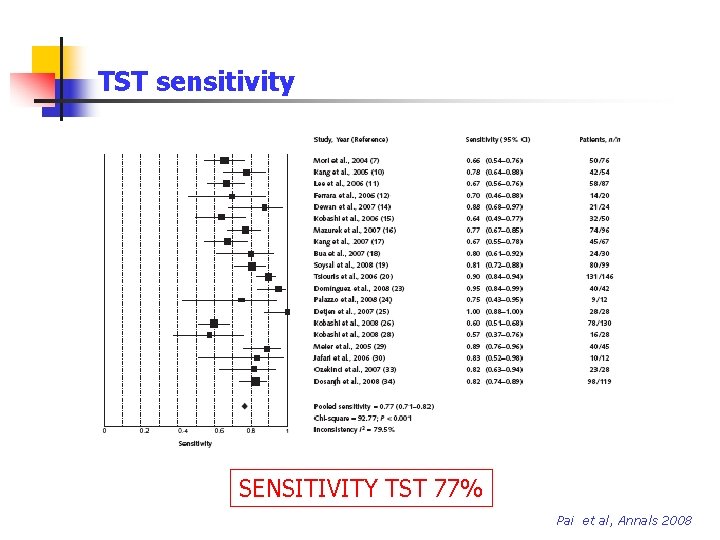
TST sensitivity SENSITIVITY TST 77% Pai et al, Annals 2008

TST specificity SPECIFICITY TST 97% SPECIFICITY TST 59% in those BCG-vaccinated Pai et al, Annals 2008

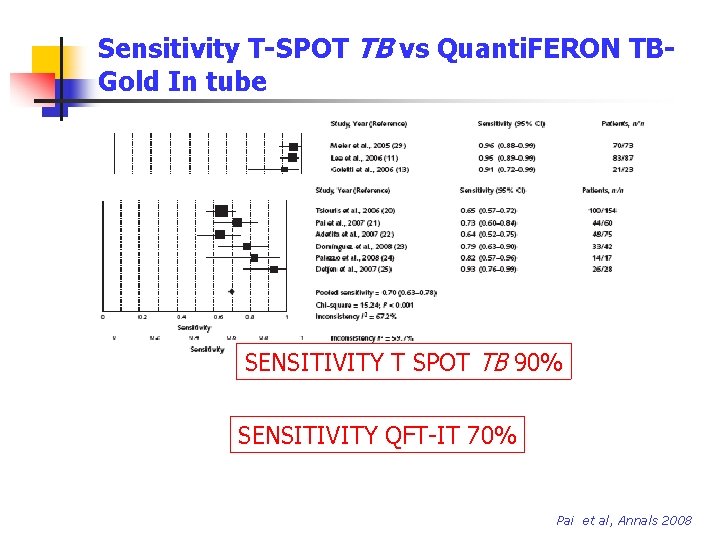
Sensitivity T-SPOT TB vs Quanti. FERON TBGold In tube SENSITIVITY T SPOT TB 90% SENSITIVITY QFT-IT 70% Pai et al, Annals 2008

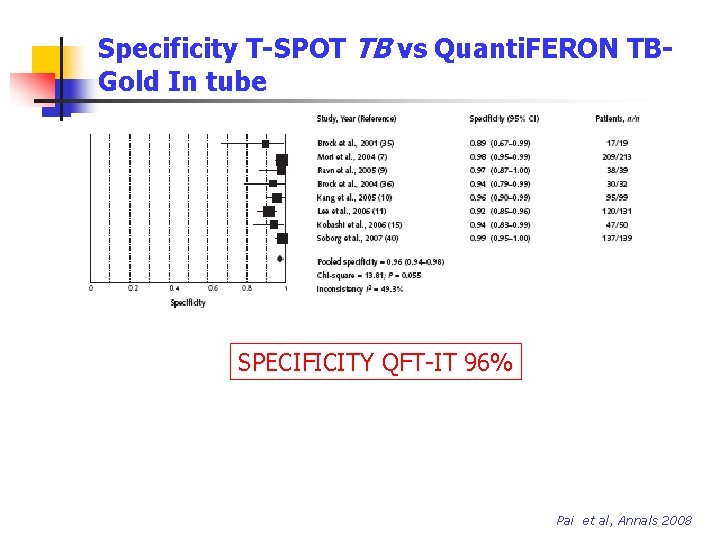
Specificity T-SPOT TB vs Quanti. FERON TBGold In tube SPECIFICITY T SPOT TB 93% SPECIFICITY QFT-IT 96% Pai et al, Annals 2008

RD 1 -IGRA Active TB disease Positive RD 1 -IGRA tuberculosis M. infection/disease Latent TB infection NTM BCG-vaccination Positive RD 1 -IGRA do not distinguish active TB disease and LTBI

Vulnerable populations n Children n Immuno-suppressed for: n n HIV Autoimmune disease

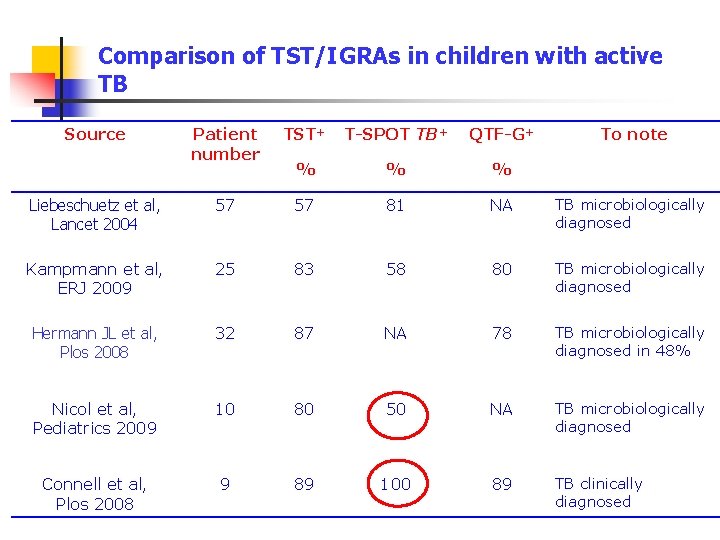
Comparison of TST/IGRAs in children with active TB Source Patient number TST+ T-SPOT TB+ QTF-G+ To note % % % Liebeschuetz et al, Lancet 2004 57 57 81 NA TB microbiologically diagnosed Kampmann et al, ERJ 2009 25 83 58 80 TB microbiologically diagnosed Hermann JL et al, Plos 2008 32 87 NA 78 TB microbiologically diagnosed in 48% Nicol et al, Pediatrics 2009 10 80 50 NA TB microbiologically diagnosed Connell et al, Plos 2008 9 89 100 89 TB clinically diagnosed

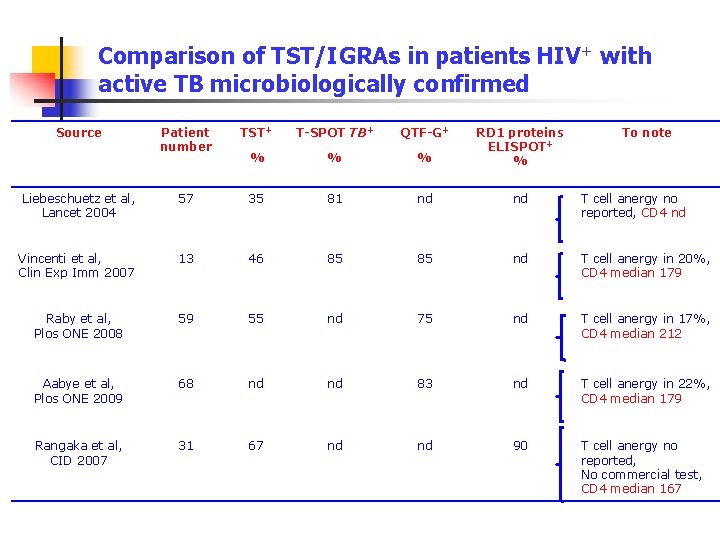
Comparison of TST/IGRAs in patients HIV+ with active TB microbiologically confirmed Source Patient number TST+ T-SPOT TB+ QTF-G+ % % % RD 1 proteins ELISPOT+ % To note Liebeschuetz et al, Lancet 2004 57 35 81 nd nd T cell anergy no reported, CD 4 nd Vincenti et al, Clin Exp Imm 2007 13 46 85 85 nd T cell anergy in 20%, CD 4 median 179 Raby et al, Plos ONE 2008 59 55 nd 75 nd T cell anergy in 17%, CD 4 median 212 Aabye et al, Plos ONE 2009 68 nd nd 83 nd T cell anergy in 22%, CD 4 median 179 Rangaka et al, CID 2007 31 67 nd nd 90 T cell anergy no reported, No commercial test, CD 4 median 167

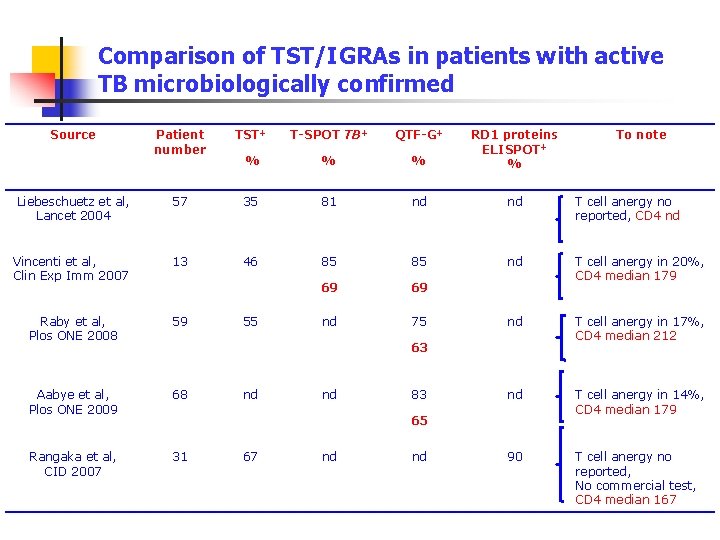
Comparison of TST/IGRAs in patients with active TB microbiologically confirmed Source Patient number TST+ T-SPOT TB+ QTF-G+ % % % RD 1 proteins ELISPOT+ % Liebeschuetz et al, Lancet 2004 57 35 81 nd nd T cell anergy no reported, CD 4 nd Vincenti et al, Clin Exp Imm 2007 13 46 85 85 nd 69 69 T cell anergy in 20%, CD 4 median 179 Raby et al, Plos ONE 2008 59 nd 75 nd T cell anergy in 17%, CD 4 median 212 Aabye et al, Plos ONE 2009 68 nd T cell anergy in 14%, CD 4 median 179 Rangaka et al, CID 2007 31 90 T cell anergy no reported, No commercial test, CD 4 median 167 55 63 nd nd 83 65 67 nd nd To note

Proportion of in vitro anergic responses to IGRAs in HIV+ patients Brock, Resp Res 2007 Vincenti, Clin Exp Imm 2007 Luetkem eyer, AJRCCM 2007 Clark, Clin Exp Imm 2007 Test QFT ELISPOT QFT N. Paz 590 111 196 201 247 84 4 (24%) 12 (57%) 5 (16%) 4 (6%) 6 (16%) 6 (46%) 100 -200 1 (3%) 4 (19%) 4 (3. 6%) 12 (31%) 3 (15%) 201 -300 5 (8%) 3 (14%) 10 (26) 3 (13%) 10 (2%) 2 (10%) 8 (21%) 4 (8%) CD 4 home-made Karam, Rabi, Plos ONE 2008 home-made per ml <100 >300 1 (NA) 6 (3. 9%)

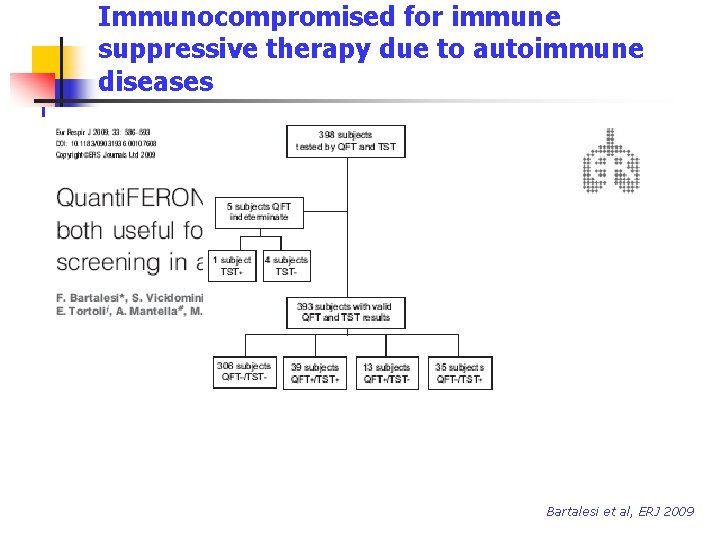
Immunocompromised for immune suppressive therapy due to autoimmune diseases Bartalesi et al, ERJ 2009

Predictive value of IGRA: HIV-negative subjects Diel et al, AJRCCM 2009

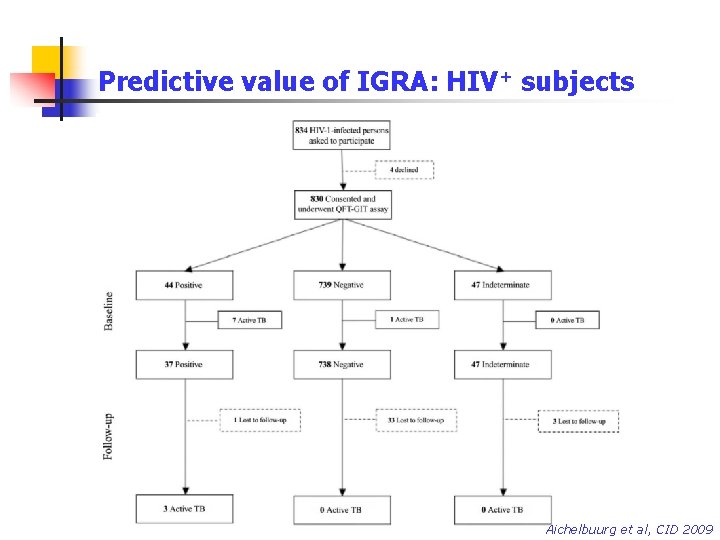
Predictive value of IGRA: HIV+ subjects Aichelbuurg et al, CID 2009

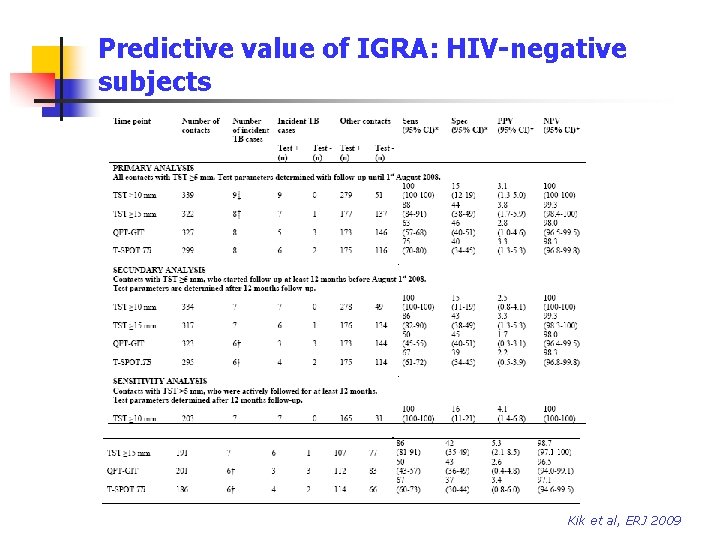
Predictive value of IGRA: HIV-negative subjects Kik et al, ERJ 2009

Need of… n Standardized test (laboratory test) n M. tuberculosis-specific reagents n Possibility to discriminate between the different stages of tuberculosis

Agenda n Problems in the diagnosis of TB n TST n IGRA n n Quanti. FERON-TB RD 1 -based assays: n n n T-SPOT TB Quanti. FERON-TB Gold New experimental tests n n n IGRA based on RD 1 selected peptides or on antigens of latency IP-10 detection IGRA at the site of TB disease

Why is it important to distinguish between latent infection and active TB disease? n To provide a correct diagnosis: n Active TB disease: n n Latent infection To provide a correct and efficacious therapy: n n n Organ destruction and/or death Spread of infection in the community Active TB disease: 2 months therapy with 4 drugs and the 2 months therapy with 2 drugs Latent TB infection: 6 months therapy with one drug To save human and economic costs avoiding complex evaluations (i. e. clinical, radiological and surgery procedures). Ex: extra-pulmonary TB

Our approach: use of peptides from ESAT-6 and CFP-10 selected by computational analysis Peptide Position sequence DR-serological specificities covered 1 - ESAT-6 6 -28 1, 3, 4, 8, 11(5), 13(6), 52, 53 2 - ESAT-6 66 -78 3, 8, 11(5), 13(6), 15(2), 52 3 - CFP-10 18 -31 3, 5, 11(5), 52 4 - CFP-10 43 -70 1, 3, 4, 7, 8, 11(5), 13(6), 15(2), 52 5 - CFP-10 74 -86 3, 4, 7, 11(5), 12(5), 13(6), 15 (2) Peptides selected by computational analysis that cover more than 90% of the HLA class II specificities

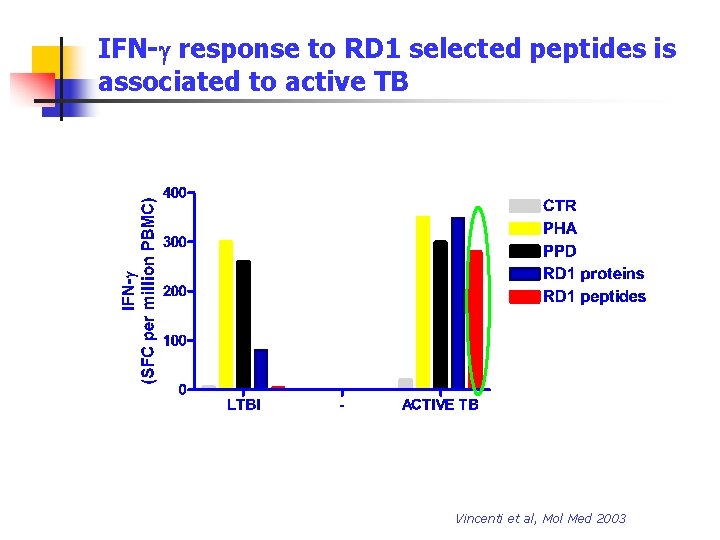
IFN-g response to RD 1 selected peptides is associated to active TB Vincenti et al, Mol Med 2003

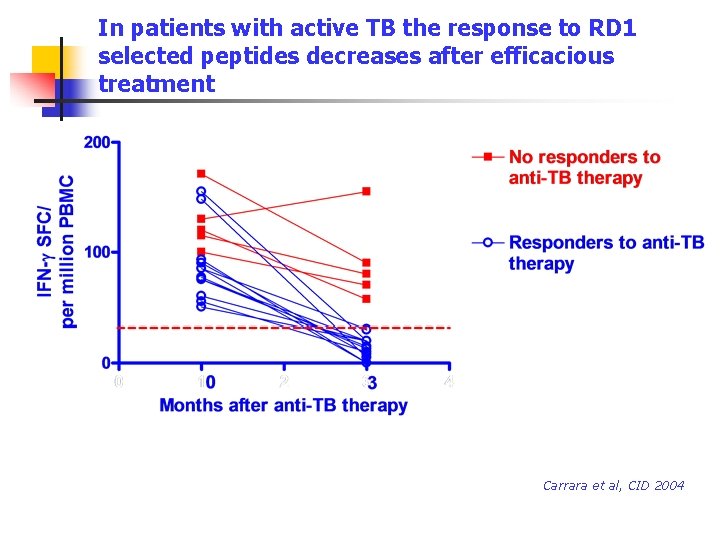
In patients with active TB the response to RD 1 selected peptides decreases after efficacious treatment Carrara et al, CID 2004

Sensitivity, specificity and diagnostic odds ratio of the different assays for the immune diagnosis of TB Assay RD 1 ELISPOT assays Sensitivity Specificity % % Selected peptides 70 91 22 Intact proteins 83 56 6 91 59 15 83 59 7 RD 1 commercially T-SPOT TB available assays QFT Gold Diagnostic odds ratio Goletti et al, CMI 2006

TBNET report Goletti et al, PLo. S ONE 2008

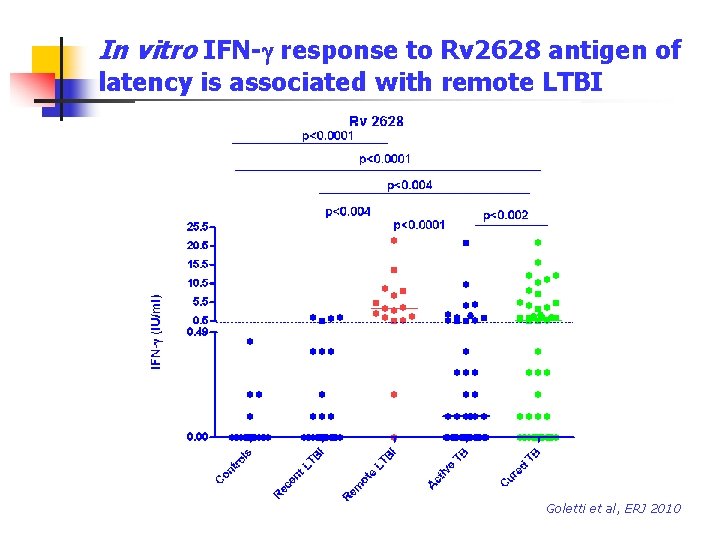
In vitro IFN-g response to Rv 2628 antigen of latency is associated with remote LTBI Goletti et al, ERJ 2010

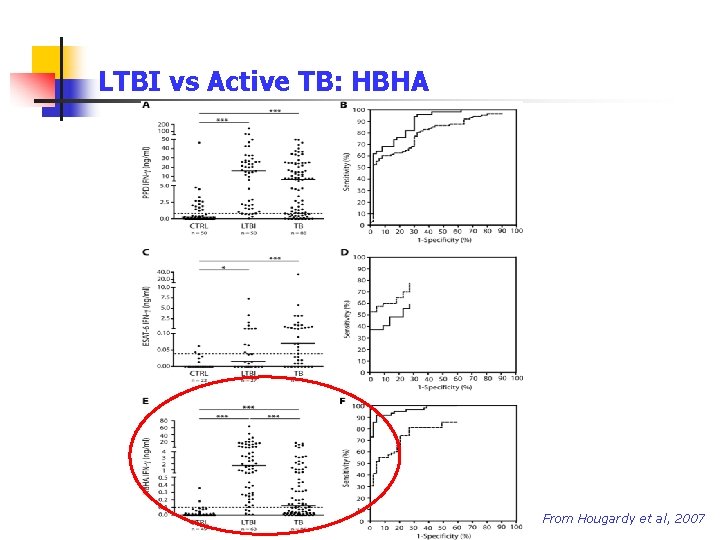
LTBI vs Active TB: HBHA Berlin, October 4 th, 2008 From Hougardy et al, 2007

Agenda n Problems in the diagnosis of TB n TST n IGRA n n Quanti. FERON-TB RD 1 -based assays: n n n T-SPOT TB Quanti. FERON-TB Gold New experimental tests n n n IGRA based on RD 1 selected peptides or on antigens of latency IGRA at the site of TB disease IP-10 detection

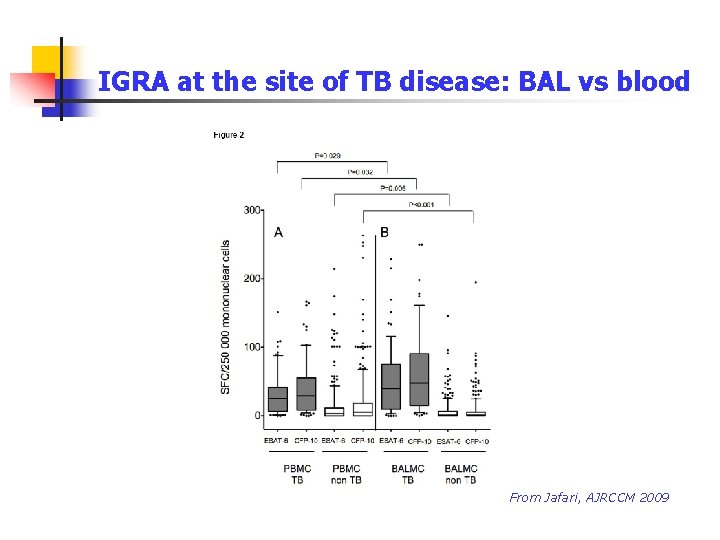
IGRA at the site of TB disease: BAL vs blood From Jafari, AJRCCM 2009

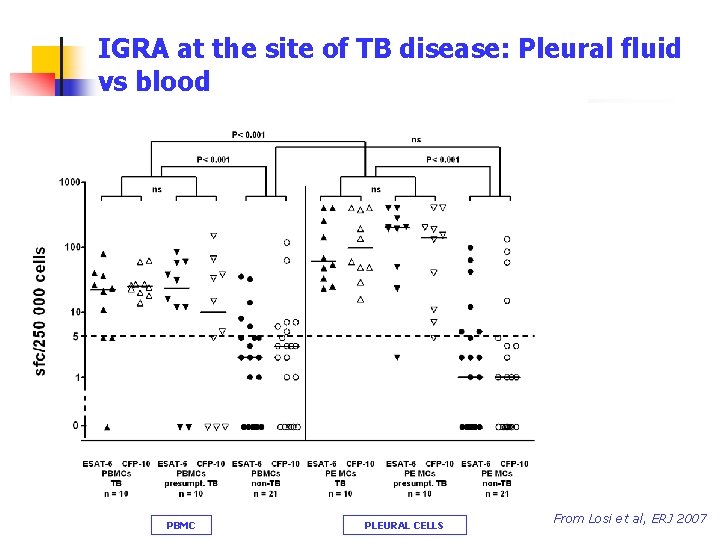
IGRA at the site of TB disease: Pleural fluid vs blood PBMC Berlin, October 4 th, 2008 PLEURAL CELLS From Losi et al, ERJ 2007

Agenda n Problems in the diagnosis of TB n TST n IGRA n n Quanti. FERON-TB RD 1 -based assays: n n n T-SPOT TB Quanti. FERON-TB Gold New experimental tests n n n IGRA based on RD 1 selected peptides or on antigens of latency IGRA at the site of TB disease IP-10 detection

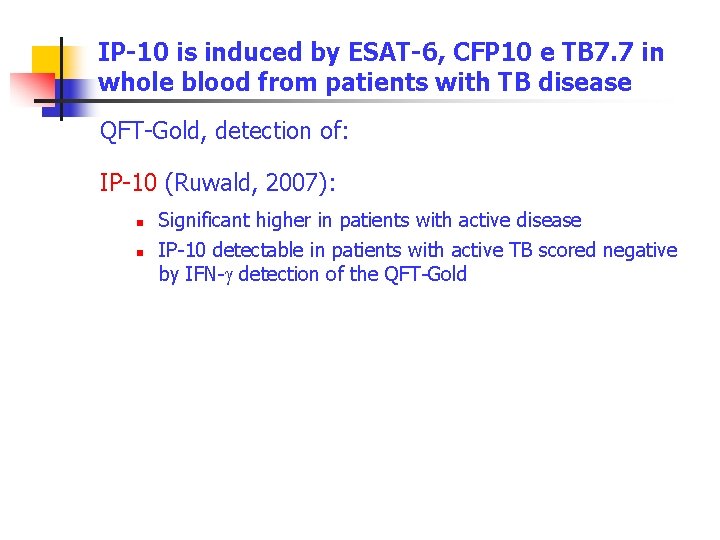
IP-10 is induced by ESAT-6, CFP 10 e TB 7. 7 in whole blood from patients with TB disease QFT-Gold, detection of: IP-10 (Ruwald, 2007): n n Significant higher in patients with active disease IP-10 detectable in patients with active TB scored negative by IFN-g detection of the QFT-Gold

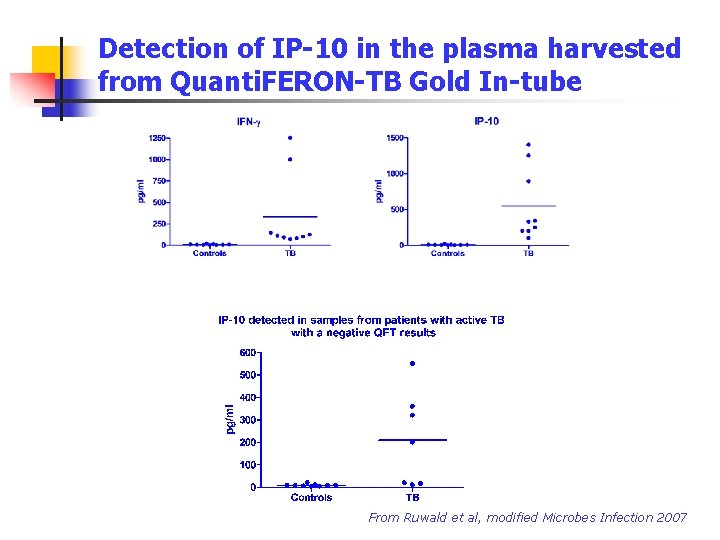
Detection of IP-10 in the plasma harvested from Quanti. FERON-TB Gold In-tube From Ruwald et al, modified Microbes Infection 2007

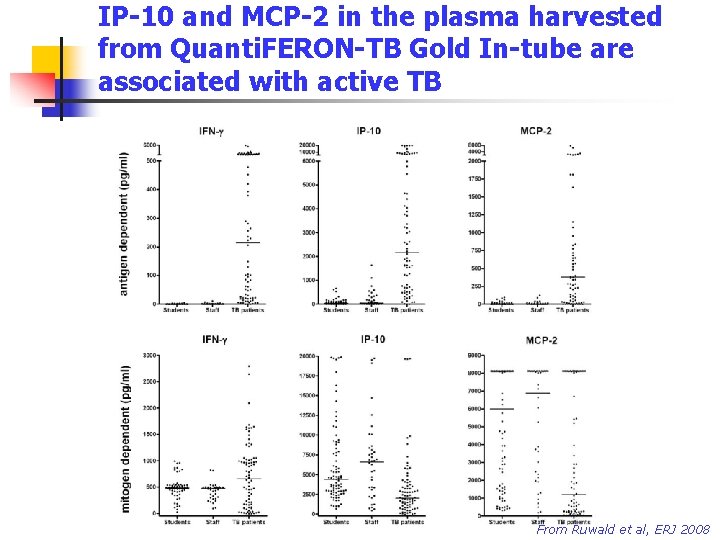
IP-10 and MCP-2 in the plasma harvested from Quanti. FERON-TB Gold In-tube are associated with active TB From Ruwald et al, ERJ 2008

Agenda n Problems in the diagnosis of TB n TST n IGRA n n Quanti. FERON-TB RD 1 -based assays: n n n T-SPOT TB Quanti. FERON-TB Gold New experimental tests n n n IGRA based on RD 1 selected peptides or on antigens of latency IGRA at the site of TB disease IP-10 detection

Agenda n Problems in the diagnosis of TB n TST n IGRA n n Quanti. FERON-TB RD 1 -based assays: n n n T-SPOT TB Quanti. FERON-TB Gold New experimental tests n n n IGRA based on RD 1 selected peptides or on antigens of latency IP-10 detection IGRA at the site of TB disease

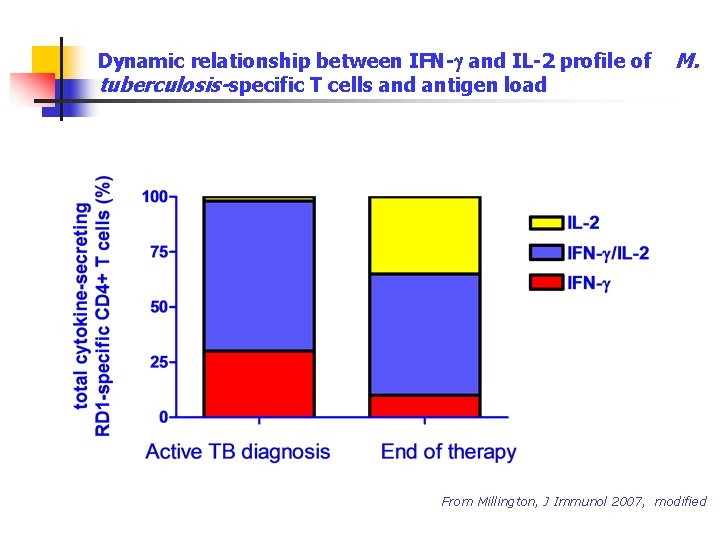
Dynamic relationship between IFN-g and IL-2 profile of tuberculosis-specific T cells and antigen load M. From Millington, J Immunol 2007, modified

Agenda n Problems in the diagnosis of TB n TST n IGRA n n Quanti. FERON-TB RD 1 -based assays: n n n T-SPOT TB Quanti. FERON-TB Gold New experimental tests n n n IGRA based on RD 1 selected peptides or on antigens of latency IP-10 detection IGRA at the site of TB disease

And thank you to:
 Flogosi infettive
Flogosi infettive Istituto comprensivo spallanzani
Istituto comprensivo spallanzani Istituto nazionale di urbanistica
Istituto nazionale di urbanistica Istituto gemmologico nazionale
Istituto gemmologico nazionale Malattie croniche
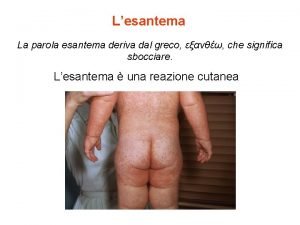
Malattie croniche Enantematico
Enantematico Malattie epifitiche
Malattie epifitiche Malattie croniche
Malattie croniche Cromatidio
Cromatidio Malattie
Malattie Malattie multifattoriali esempi
Malattie multifattoriali esempi Malattie cardiovascolari
Malattie cardiovascolari Malattie dismielinizzanti
Malattie dismielinizzanti Malattie epifitiche
Malattie epifitiche Kousmine malattie autoimmuni
Kousmine malattie autoimmuni Fioretta del vino
Fioretta del vino Lazzaro spallanzani año de su experimento
Lazzaro spallanzani año de su experimento John needham experiment
John needham experiment Manipulated variable definition
Manipulated variable definition Generacion espontanea
Generacion espontanea Spallanzani experiment
Spallanzani experiment Teori asal usul kehidupan disusun berdasarkan …
Teori asal usul kehidupan disusun berdasarkan … Teoria de miller
Teoria de miller Experimentos de redi spallanzani y pasteur
Experimentos de redi spallanzani y pasteur Spallanzani experiment
Spallanzani experiment Bab 7 evolusi
Bab 7 evolusi Spallanzani
Spallanzani Pescoço de cisne pasteur
Pescoço de cisne pasteur Teori kataklisma
Teori kataklisma Carta nazionale delle professioni museali
Carta nazionale delle professioni museali Inno di mameli da stampare
Inno di mameli da stampare Piano nazionale scuola digitale azioni
Piano nazionale scuola digitale azioni Enac ente nazionale canossiano
Enac ente nazionale canossiano Classificazione nazionale dei dispositivi medici
Classificazione nazionale dei dispositivi medici Anbba
Anbba Asl novara
Asl novara Centro nazionale trapianti
Centro nazionale trapianti Modello nazionale di certificazione delle competenze
Modello nazionale di certificazione delle competenze Festa nazionale inghilterra
Festa nazionale inghilterra Borsa continua del lavoro
Borsa continua del lavoro Direzione nazionale antimafia organigramma
Direzione nazionale antimafia organigramma Classificazione cnd dispositivi medici
Classificazione cnd dispositivi medici Registro nazionale società sportive dilettantistiche
Registro nazionale società sportive dilettantistiche Centro nazionale trapianti
Centro nazionale trapianti Rilab data sc
Rilab data sc Rete accelerometrica nazionale
Rete accelerometrica nazionale Sport nazionale tedesco
Sport nazionale tedesco Consolidato mondiale
Consolidato mondiale Direzione nazionale antimafia e antiterrorismo
Direzione nazionale antimafia e antiterrorismo Parafrasi inno mameli
Parafrasi inno mameli Federazione opere educative
Federazione opere educative Tesori da scoprire fidapa
Tesori da scoprire fidapa Centro nazionale trapianti
Centro nazionale trapianti Rete accelerometrica nazionale
Rete accelerometrica nazionale Galleria nazionale delle marche
Galleria nazionale delle marche Il reddito nazionale
Il reddito nazionale Primi tentativi di educazione dei ciechi
Primi tentativi di educazione dei ciechi Livia bottardi
Livia bottardi Longum iter est
Longum iter est Una voce il mio diletto
Una voce il mio diletto 20 hm/menit berapa m/menit
20 hm/menit berapa m/menit Per capita vs per stirpes
Per capita vs per stirpes Laukas nematomas, avys neskaitytos, piemuo raguotas.
Laukas nematomas, avys neskaitytos, piemuo raguotas. Approssimazione per difetto e per eccesso
Approssimazione per difetto e per eccesso Per stirpes v per capita
Per stirpes v per capita Catullus 84
Catullus 84 186 282 miles per second into meters per second
186 282 miles per second into meters per second Coop per me e per te
Coop per me e per te Multas per gentes et multa per aequora vectus
Multas per gentes et multa per aequora vectus