ISOTOPIC NOTATION isotopes are atoms with the same





- Slides: 15

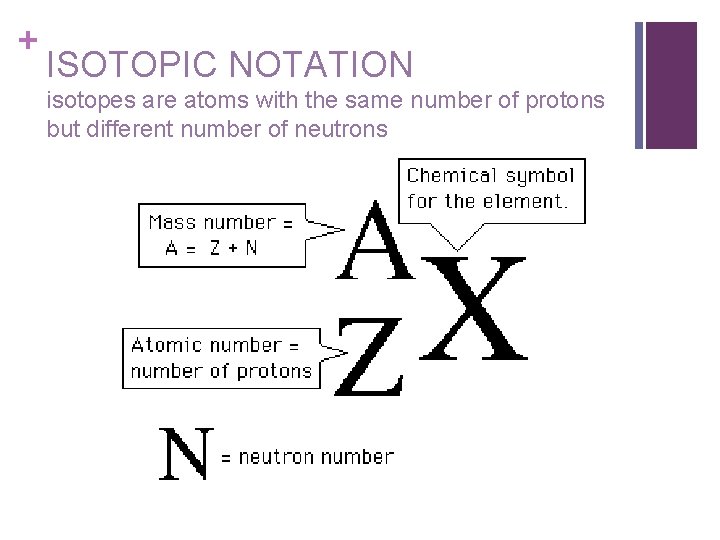
+ ISOTOPIC NOTATION isotopes are atoms with the same number of protons but different number of neutrons


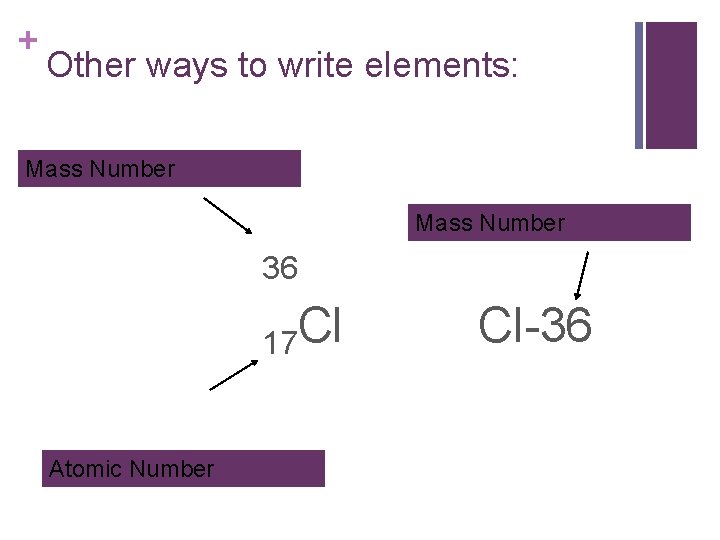
+ Other ways to write elements: Mass Number 36 17 Cl Atomic Number Cl-36

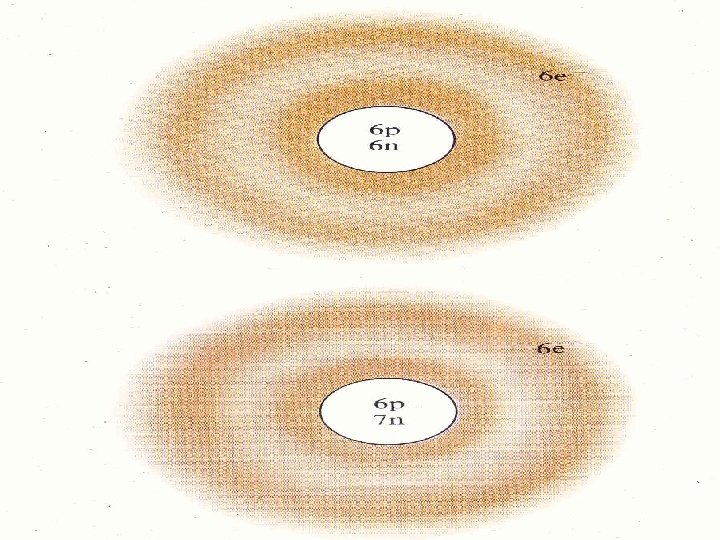
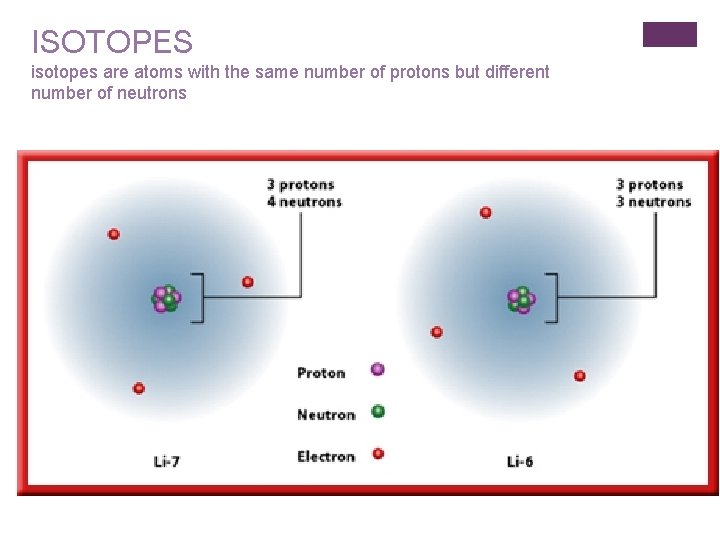
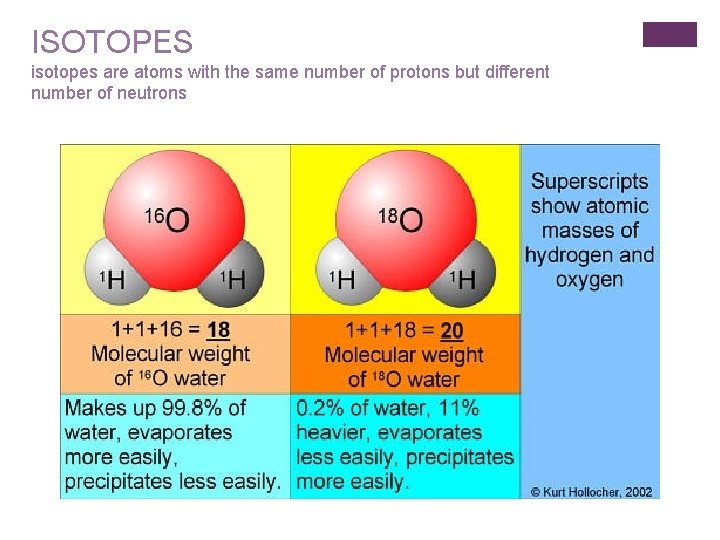
ISOTOPES isotopes are atoms with the same number of protons but different number of neutrons

ISOTOPES isotopes are atoms with the same number of protons but different number of neutrons

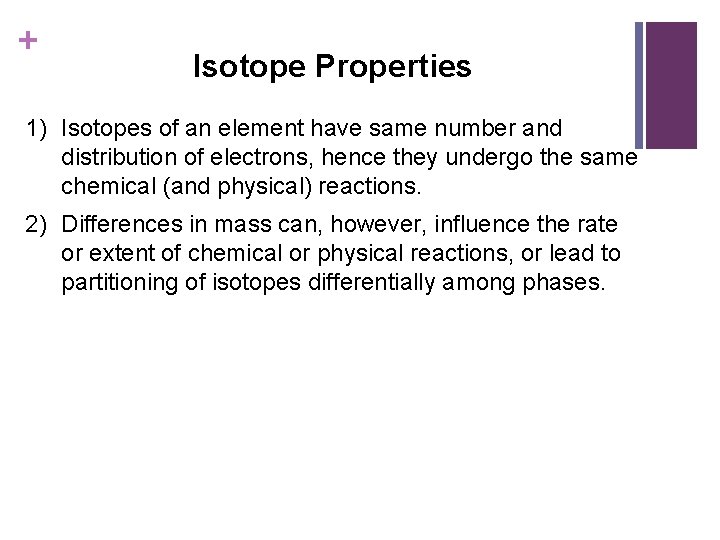
+ Isotope Properties 1) Isotopes of an element have same number and distribution of electrons, hence they undergo the same chemical (and physical) reactions. 2) Differences in mass can, however, influence the rate or extent of chemical or physical reactions, or lead to partitioning of isotopes differentially among phases.

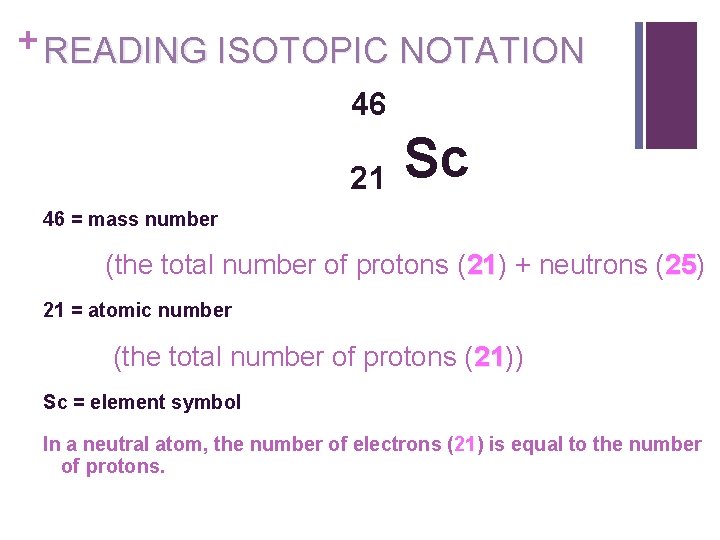
+ READING ISOTOPIC NOTATION 46 21 Sc 46 = mass number (the total number of protons (21) 21 + neutrons (25) 25 21 = atomic number (the total number of protons (21)) 21 Sc = element symbol In a neutral atom, the number of electrons (21) is equal to the number of protons.

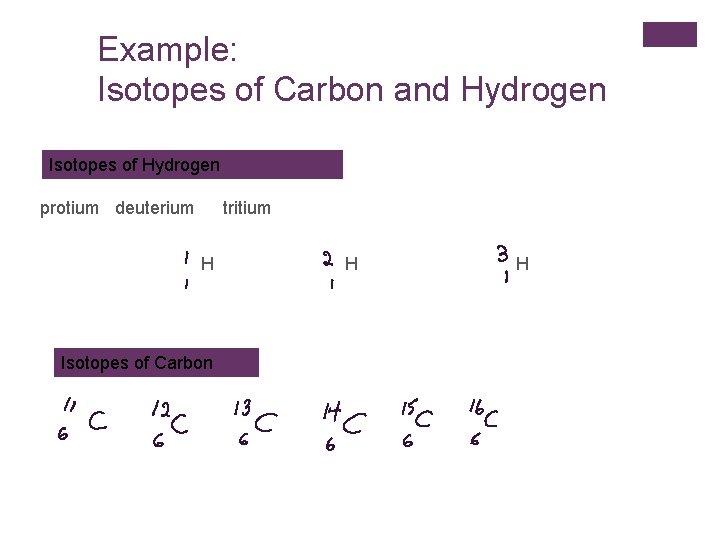
Example: Isotopes of Carbon and Hydrogen Isotopes of Hydrogen protium deuterium tritium H Isotopes of Carbon H H

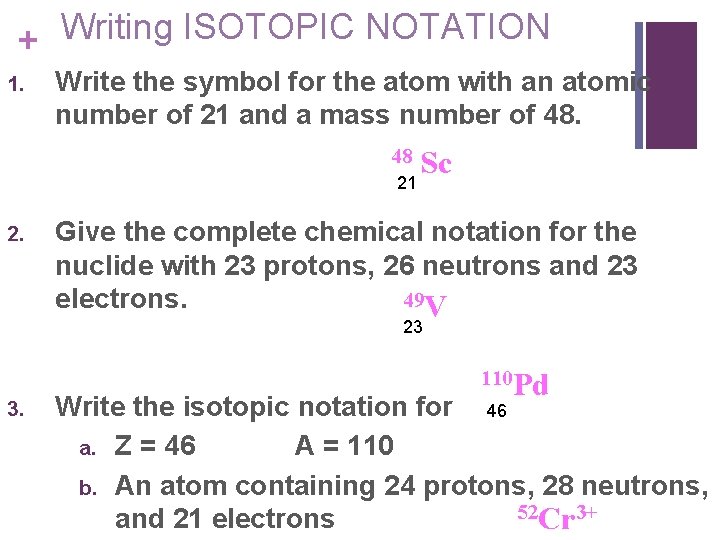
Writing ISOTOPIC NOTATION + 1. Write the symbol for the atom with an atomic number of 21 and a mass number of 48. 48 21 2. Sc Give the complete chemical notation for the nuclide with 23 protons, 26 neutrons and 23 49 V electrons. 23 3. 110 Pd Write the isotopic notation for 46 a. Z = 46 A = 110 b. An atom containing 24 protons, 28 neutrons, 52 Cr 3+ and 21 electrons

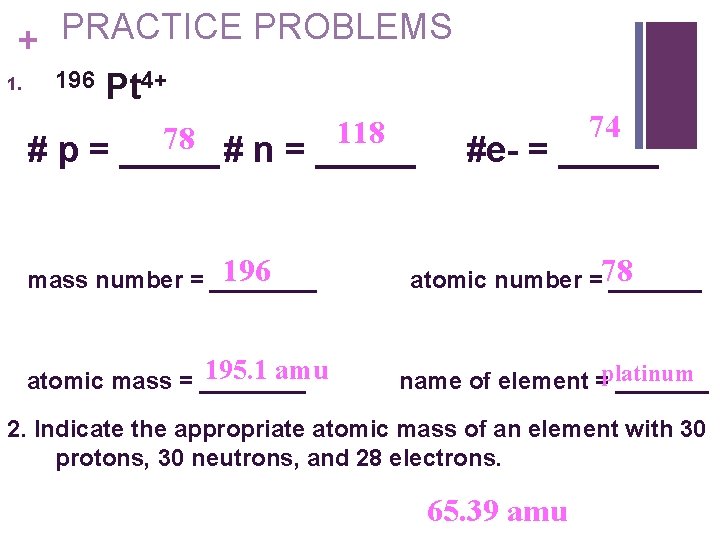
PRACTICE PROBLEMS + 196 Pt 4+ 1. 118 78 # p = _____# n = _____ 74 #e- = _____ 196 mass number = ____ atomic number =78 _______ 195. 1 amu atomic mass = ____ name of element =platinum _______ 2. Indicate the appropriate atomic mass of an element with 30 protons, 30 neutrons, and 28 electrons. 65. 39 amu


Atomic Mass n The atomic mass of an element represents the average mass of all the isotopes found in nature. No element exists with only one possible isotope. Hydrogen has the smallest number of isotopes: 1 H protium, 2 H deuterium, 3 H tritium. Its atomic mass is 1. 0079 amu (atomic mass units). The atomic mass is calculated by adding the % of 1 H mass found in nature to the % of 2 H mass found in nature plus the % of 3 H mass. n % 1 H + % 2 H + % 3 H = average mass (atomic mass) n Generally the formula used is: % X + % Y + % Z… = atomic mass. An instrument called the mass spectrometer is generally used to determine the percentages and individual masses of each isotope.

Atomic Mass n Silver is found to have two stable isotopes, one has an atomic mass of 106. 904 amu and the other weighs 108. 905 amu. The first isotope represents 51. 82 % of the mass of the element and the second represents 48. 18 %. What is the atomic mass of the element silver? The equation to use is %X + % Y = average And remember to turn your percents into fractions before multiplying. (0. 5182) 106. 904 amu + (0. 4818) 108. 905 amu =? 55. 398 amu + 52. 470 amu =? 107. 868 amu !! Now look at the periodic table to verify the answer.

PRACTICE PROBLEMS # 8 1. A sample of neon contains three isotopes, neon-20 (with an isotopic mass of 19. 9924 amu), neon-21 (20. 9939 amu) and neon-22 (21. 9914 amu). The natural abundances of these isotopes are 90. 92%, 0. 257 %, and 8. 82 %. Calculate the atomic weight of neon. 20. 17 amu 2. There are only two naturally occuring isotopes of copper, 63 Cu and 65 Cu. Copper has an atomic mass of 63. 55 amu. What is the natural abundance of each isotope? 65 63 Cu = 30% & Cu = 70% 3. There are only two naturally occuring isotopes of gallium, 69 Ga and 71 Ga. What is the natural abundance 69 Ga = 60% and 71 Ga = 40% of each isotope?

GROUP STUDY PROBLEM #8 What is the relative atomic mass of boron if two stable isotopes of boron have the following mass and abundance: 10. 0129 amu (19. 91%) & 11. 0129 (80. 09%) a) 10. 81 amu b) 10. 21 amu c) 10. 62 amu d) 10. 51 amu