Isotopic Abundance Weighted Average Atomic Mass Isotopes l

Isotopic Abundance & Weighted Average Atomic Mass

Isotopes l Atoms with the same number of protons, but different numbers of neutrons. l Atoms of the same element (same atomic number) with different mass numbers Isotopes of chlorine 35 Cl 37 Cl 17 17 chlorine 35 chlorine 37
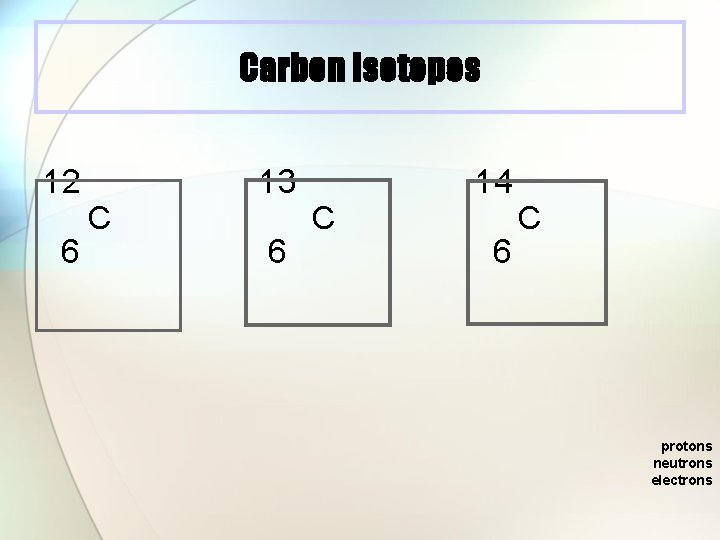
Carbon Isotopes 12 6 C 13 6 C 14 6 C protons neutrons electrons
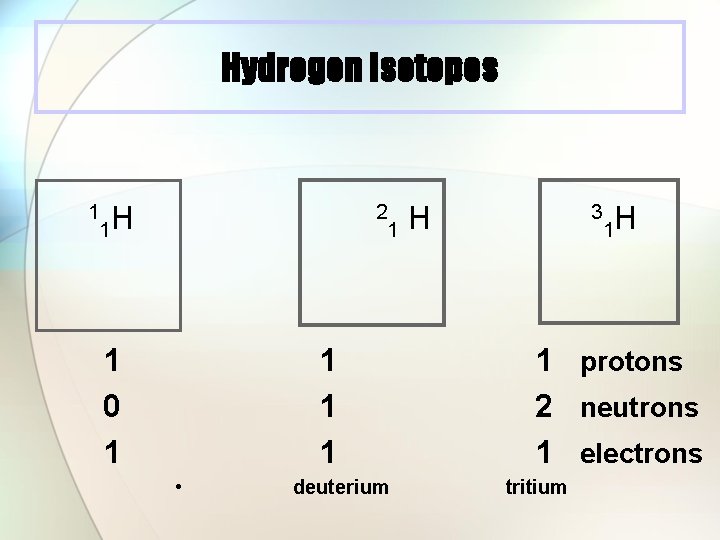
Hydrogen Isotopes 1 1 H 2 1 0 1 1 H 1 1 1 • deuterium 3 1 H 1 protons 2 neutrons 1 electrons tritium
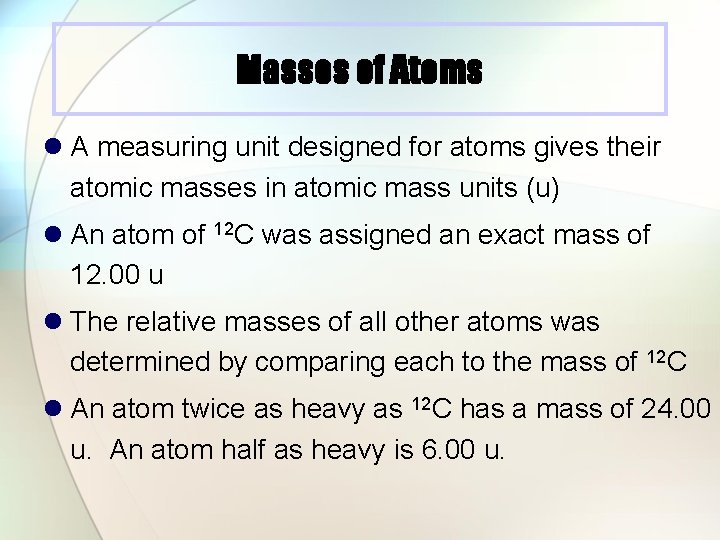
Masses of Atoms l A measuring unit designed for atoms gives their atomic masses in atomic mass units (u) l An atom of 12 C was assigned an exact mass of 12. 00 u l The relative masses of all other atoms was determined by comparing each to the mass of 12 C l An atom twice as heavy as 12 C has a mass of 24. 00 u. An atom half as heavy is 6. 00 u.
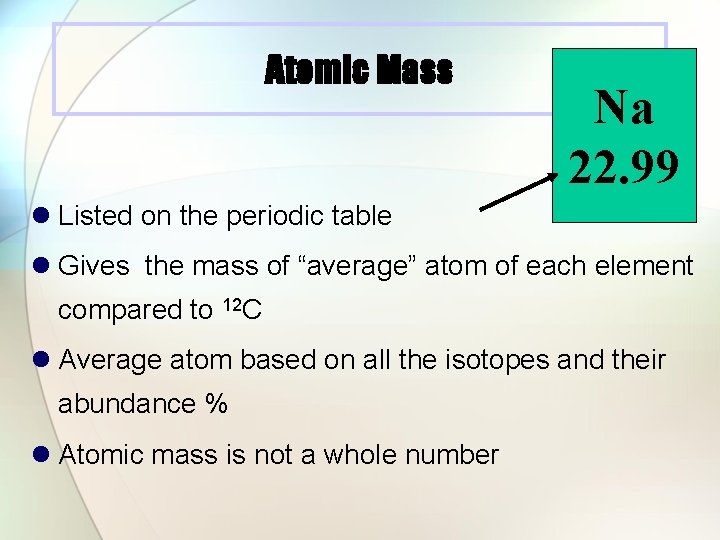
Atomic Mass Na 22. 99 l Listed on the periodic table l Gives the mass of “average” atom of each element compared to 12 C l Average atom based on all the isotopes and their abundance % l Atomic mass is not a whole number
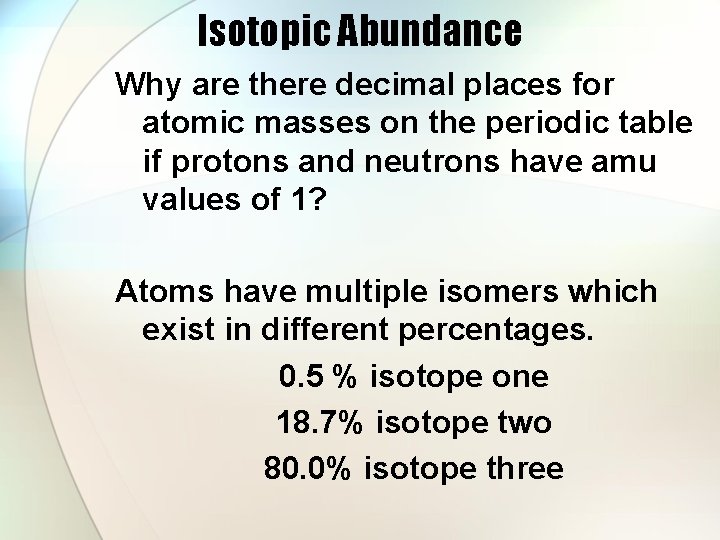
Isotopic Abundance Why are there decimal places for atomic masses on the periodic table if protons and neutrons have amu values of 1? Atoms have multiple isomers which exist in different percentages. 0. 5 % isotope one 18. 7% isotope two 80. 0% isotope three

Isotopic Abundance The values on the periodic table represent the “average” mass of any single atom for the element.

Isotopic Abundance Problems Two main types: 1. Find the “average” mass given the percentages. 2. Find the percentages given the “average” mass.

Weighted Average • The values on the periodic table are average atomic masses. • Average atomic mass is the atomic mass of the mixture of isotopes of an element that is usually found in nature. e. g. AZX, BZX, CZX …. • Average atomic mass = (% AZX * mass AZX) + (% BZX * mass BZX)

• Chlorine has two naturally occurring isotopes, namely and. The composition of chlorine is 25. 0% and 75. 0%. The average atomic mass of chlorine is • ANSWER: 35. 5 u

Example #1 Given: mass of 7 Li = 7. 015 u mass of 6 Li = 6. 015 u Of all existing Li atoms, 92. 58% are 7 Li, while the remaining is 6 Li. What is the “average” mass of an Li atom?

Example #2 In nature, silicon is composed of three isotopes. Calculate the average atomic mass of silicon given: 28 Si (92. 23%, 27. 98 u) 29 Si (4. 67%, 28. 97 u) 30 Si (3. 10%, 29. 97 u)
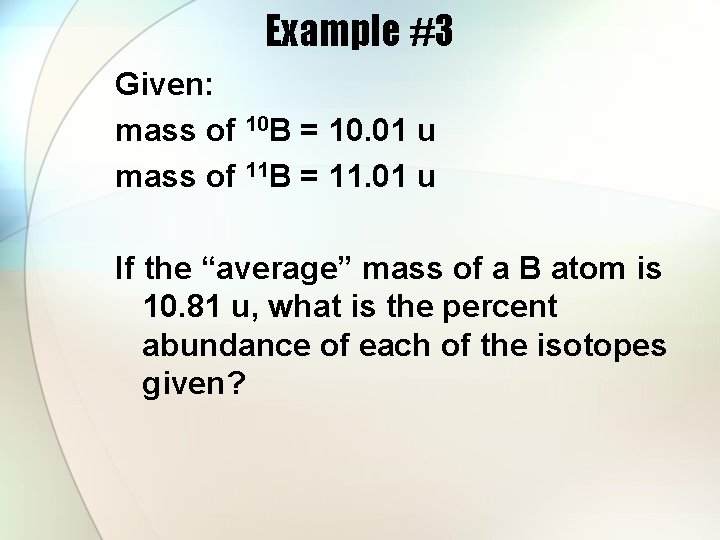
Example #3 Given: mass of 10 B = 10. 01 u mass of 11 B = 11. 01 u If the “average” mass of a B atom is 10. 81 u, what is the percent abundance of each of the isotopes given?

Learning Check! Gallium is a metallic element found in small lasers used in compact disc players. In a sample of gallium, there is 60. 2% of gallium-69 (68. 9 u) atoms and 39. 8% of gallium 71 (70. 9 u) atoms. What is the atomic mass of gallium?

Finding An Isotopic Mass A sample of boron consists of 10 B (mass 10. 0 u) and 11 B (mass 11. 0 u). If the average atomic mass of B is 10. 8 u, what is the % abundance of each boron isotope?
- Slides: 16