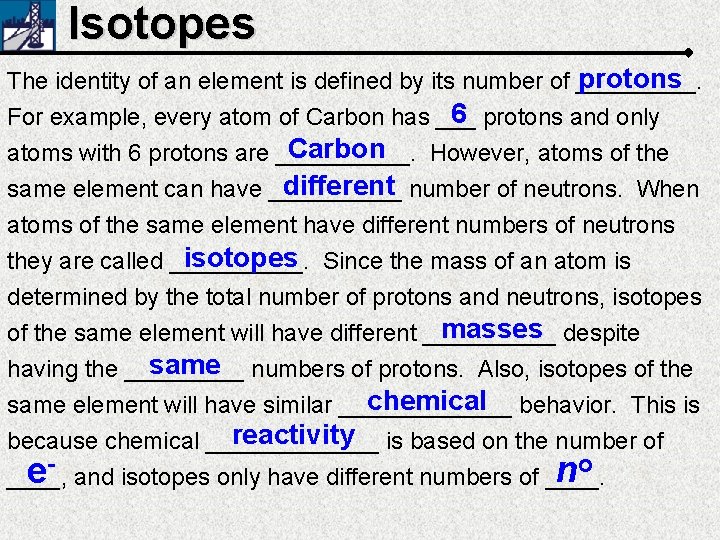
Isotopes protons The identity of an element is
- Slides: 6

Isotopes protons The identity of an element is defined by its number of _____. 6 protons and only For example, every atom of Carbon has ___ Carbon atoms with 6 protons are _____. However, atoms of the different number of neutrons. When same element can have _____ atoms of the same element have different numbers of neutrons isotopes Since the mass of an atom is they are called _____. determined by the total number of protons and neutrons, isotopes masses despite of the same element will have different _____ same numbers of protons. Also, isotopes of the having the _____ chemical behavior. This is same element will have similar _______ reactivity is based on the number of because chemical _______ o n e ____, and isotopes only have different numbers of ____.

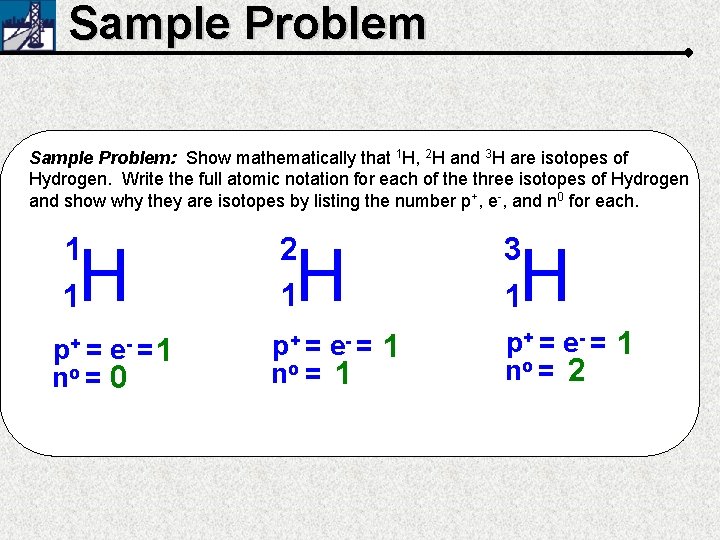
Sample Problem: Show mathematically that 1 H, 2 H and 3 H are isotopes of Hydrogen. Write the full atomic notation for each of the three isotopes of Hydrogen and show why they are isotopes by listing the number p+, e-, and n 0 for each. 1 1 2 1 H p+ = e- = no = 0 H 1 p+ = e - = 1 no = 1 3 1 H p+ = e - = 1 no = 2

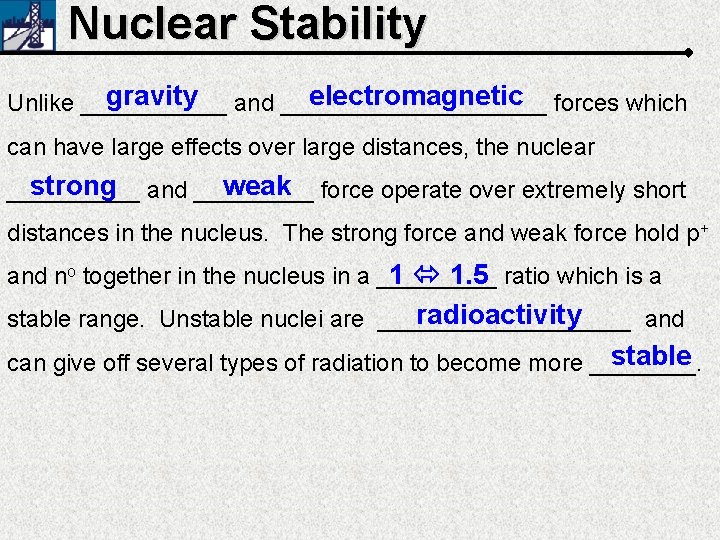
Nuclear Stability gravity and __________ electromagnetic forces which Unlike ______ can have large effects over large distances, the nuclear strong and _____ weak force operate over extremely short _____ distances in the nucleus. The strong force and weak force hold p+ 1 1. 5 ratio which is a and no together in the nucleus in a _____ radioactivity stable range. Unstable nuclei are __________ and stable can give off several types of radiation to become more ____.

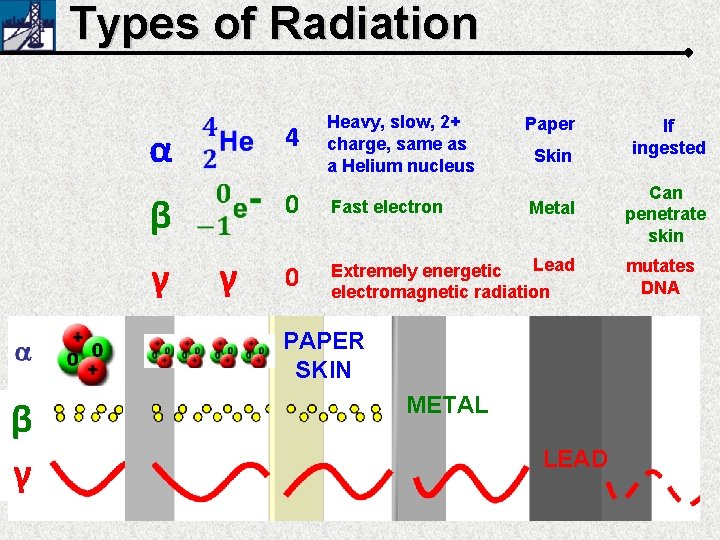
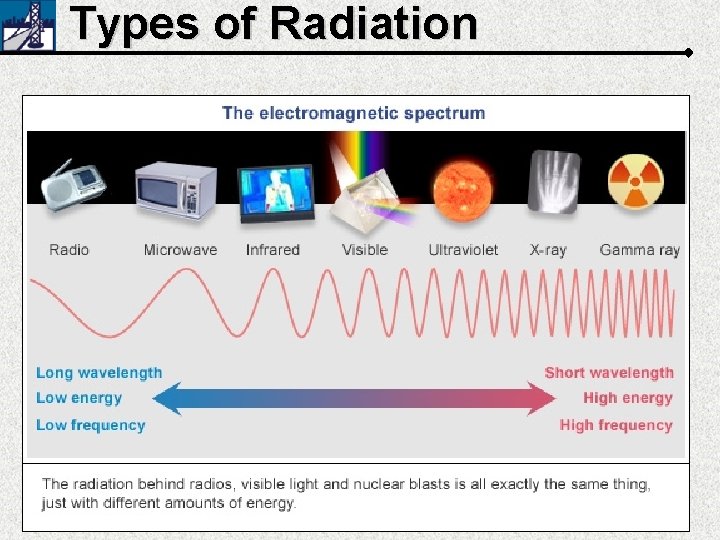
Types of Radiation 4 α β γ γ Heavy, slow, 2+ charge, same as a Helium nucleus Paper Skin 0 Fast electron 0 Lead Extremely energetic electromagnetic radiation Metal PAPER SKIN β γ METAL LEAD If ingested Can penetrate skin mutates DNA

Types of Radiation

Chemical vs. Nuclear Changes While chemical changes can break compounds into their more basic elements or create more complicated molecules out of decay simpler elements, in nuclear changes like radioactive _____ elements nuclei actually change into different more stable _______. greater amounts of Also, nuclear changes release much ______ chemical changes. energy than ______