Isotopes and Relative Atomic Mass Learning Objectives Do

Isotopes and Relative Atomic Mass Learning Objectives: Do I know… • about the relative atomic mass and isotopes? • how to calculate relative atomic mass from percentage abundance? Stick in the Key Words sheet you have been given.
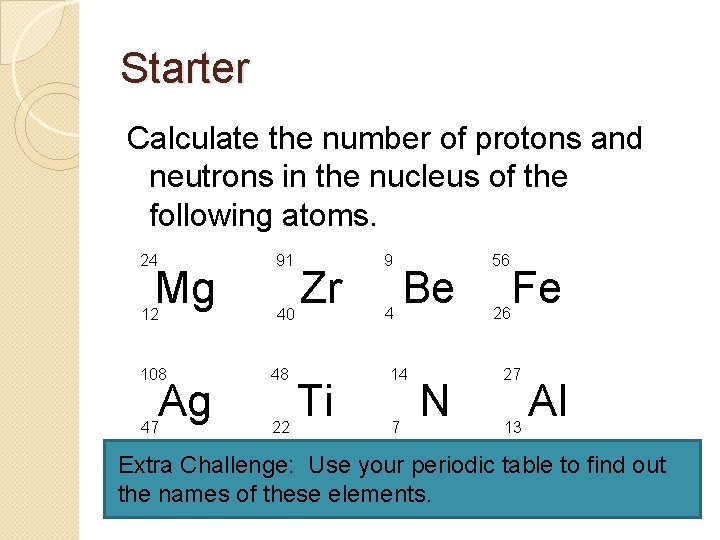
Starter Calculate the number of protons and neutrons in the nucleus of the following atoms. 24 91 12 40 Mg 108 48 47 22 Ag Zr Ti 9 4 Be 14 7 N 56 26 Fe 27 13 Al Extra Challenge: Use your periodic table to find out the names of these elements.

How did you do? 1. 2. 3. 4. 5. 6. 7. 8. Mg = 12 protons, 12 neutrons (Magnesium) Zr = 40 protons, 51 neutrons (Zirconium) Be = 4 protons, 5 neutrons (Beryllium) Fe = 26 protons, 30 neutrons (Iron) Ag = 47 protons, 61 neutrons (Silver) Ti = 22 protons, 26 neutrons (Titanium) N = 7 protons, 7 neutrons (Nitrogen) Al = 13 protons, 14 neutrons

Two kinds of carbon atoms? Almost all the carbon atoms found in living things, and in the rocks, seas and atmosphere of our planet have C the nuclear symbol. This is called Carbon-12. How many neutrons does Carbon-12 have in its nucleus? 12 6

Two kinds of carbon atoms? A tiny fraction of the carbon atoms on Earth have the nuclear symbol. C This is known as Carbon-14. 14 6 How many neutrons does Carbon-14 have in its nucleus? What are the mass numbers of Carbon 12, and Carbon-14? What about the atomic numbers?

Two kinds of carbon atoms? The two different forms of the carbon atoms are called isotopes of the element carbon. There is also a third isotope of carbon with a mass number of 13. Write down the nuclear symbol for Carbon-13.

Carbon Dating Carbon-14 is radioactive. This means that when a living organism dies, the Carbon-14 atoms it contains gradually decay into more stable carbon atoms. Scientists can use this fact to find the age of the remains of long-dead organisms. This is called carbon dating. Ötzi the ‘Iceman’ was found in a glacier in the Alps. Archaeologists used the amount of Carbon-14 remaining in his body to calculate that he died about 5, 000 years ago.

Relative atomic mass The relative atomic mass we see written on the periodic table is an average. Only a tiny fraction of carbon atoms have a mass number of 13 or 14. All the rest have a mass number of 12. So, the average mass of carbon atoms is 12. 01. This is called the relative atomic mass of carbon.

Isotopes and relative atomic mass 35 The most common isotope of chlorine Cl is. But about a quarter of all chlorine atoms are a different Cl isotope. Because this is quite a high proportion, the relative atomic mass of chlorine is not a whole number – it is actually 35. 5. 17 37 17

Calculating relative atomic mass Boron has two isotopes. B , which accounts 10 5 11 for 20% of all Boron atoms, B and , which accounts for the other 80%. What is the relative atomic mass of Boron? Step 1: Multiply the mass number of each isotope by its % abundance. 10 x 20 = 200, and 11 x 80 = 880. Step 2: Add these values together. 200 + 880 = 1080. Step 3: Finally, divide by 100 to get the average. 1080 ÷ 100 = 10. 8 5
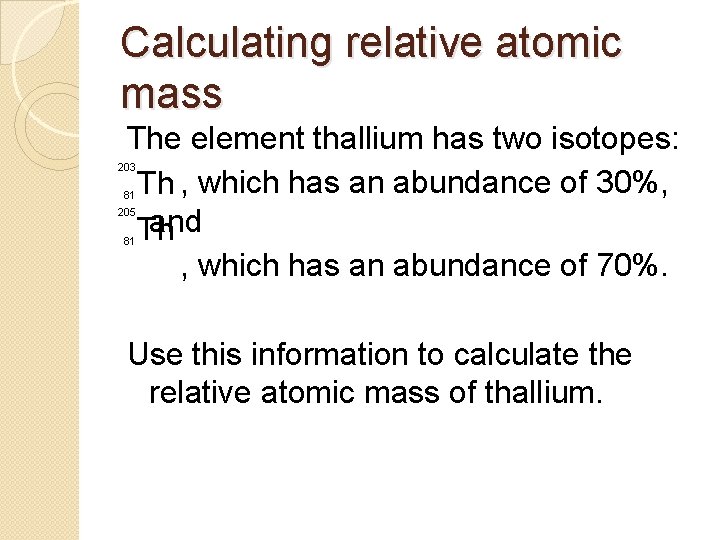
Calculating relative atomic mass The element thallium has two isotopes: Th , which has an abundance of 30%, and Th , which has an abundance of 70%. 203 81 205 81 Use this information to calculate the relative atomic mass of thallium.

Calculating relative atomic mass Thallium-203. 203 x 30 = 6090 Thallium-205. 205 x 70 = 14350 6090 + 14350 = 20440 ÷ 100 = 204. 4

Test Yourself 1. Look at the nuclear symbols for four atoms, J, X, Z and A. 14 7 J 16 8 X 15 7 Z 14 6 A How many of these atoms have 8 neutrons in their nucleus? b) Which two of these atoms are isotopes of each other? a)

Home Learning Calculate the relative atomic masses of the elements using the information printed on your sheets.
- Slides: 14