Isotope coded affinity tag ICAT Isotope coded affinity





























- Slides: 47

Isotope coded affinity tag (ICAT) Isotope coded affinity tag commonly known as ICAT is one of the advancement in the proteomics to quantify the peptides and to identify the simultaneously by mass spectrometry analysis Related LOs: Affinity tag > Prior Viewing – IDD-1. Extraction of bacterial protein, IDD-41. Liquid chromatography - Gel filtration > Future Viewing – IDD: 31. MALDI-TOF data analysis, IDD: 39. LC-MS/MS data analysis Course Name: Isotope coded affinity tag (ICAT) Level(UG/PG): PG Author(s): Dinesh Raghu, Vinayak Pachapur Mentor: Dr. Sanjeeva Srivastava *The contents in this ppt are licensed under Creative Commons Attribution-Non. Commercial-Share. Alike 2. 5 India license

1 Learning objectives • 2 3 4 5 After interacting with this learning object, the learner will be able to: 1. Define cell culture process 2. Carry out isotope tagging to peptides 3. Infer peptide enrichment by affinity column 4. Perform LC-MS/MS data and Spectrum analysis 5. Assess the troubleshooting steps involved in the experiments.

1 2 Master Layout (Part 1) Slide 20 -22 Slide 23 -34 Slide 36 -42 Light (d 0) ICAT labeled Sample 1 Slide 5 Slide 6 -20 Mixed samples Gel filteration Affinity purification 3 Heavy (d 8) ICAT labeled Sample 2 5 LC-MS/MS analysis Relative abundance 4 Slide 35 In-solution trypsin treatment Slide 43 Retention time Gygi, S. P. et al. , Quantitative analysis of complex protein mixtures using isotope-coded affinity tags. Nat Biotech. 1999, 17: 994 -999.

1 2 3 Definitions of the components: 1. Isotope Coded Affinity Tagging (ICAT): ICAT is an in vitro labeling technique that modifies peptides or proteins specifically at the cysteine amino acid residue and can be used for accurate quantitation of protein expression. 2. Light ICAT label: The light ICAT reagent consists of a Cys-reactive group, an ICAT linker consisting of hydrogen atoms and a biotin tag. The chemically reactive group forms covalent bonds with peptides or proteins while the affinity tag enables the protein to be isolated by affinity chromatography in a single step that is used to tag the control sample. 3. Heavy ICAT label: The heavy ICAT label consists of a Cys-reactive group, an ICAT linker consisting of heavy deuterium isotope and a biotin tag that is used to tag the Drug treated sample. 4. Affinity purification: A chromatographic purification procedure that makes use of specific interactions between the analyte of interest and the capture reagent immobilized on the column. In ICAT, avidin affinity chromatography is employed due to its specificity of interaction with biotin. 5. Affinity purified peptides: The peptides obtained after purification on the affinity column. 4 5 6. LC-MS/MS analysis: The peptides obtained are further purified by liquid chromatography and then analyzed by tanderm MS. Both quantity and sequence identity of proteins from which the tagged peptides were obtained can be determined. 7. Gel filtration chromatography: The peptide separation is based on the molecular weight of the protein and the molecular filtration efficiency of the gel.

1 Step 1: T 1: Culture Growth 2 3 4 5 Description of the action/ interactivity Take out master culture plate and LB broth from incubator and transfer it on laminar bench. With user control, open the culture plate, pick a colony from the master culture using the tooth pick, close the culture plate. Take the LB broth tube unplug the cotton roll with left hand. Now transfer the culture to the LB broth, by contacting the solution with the tooth pick. Please redraw the figure Audio Narration (if any) Pick a bacterial colony using the tooth pick from the master culture. Inoculate the bacterial colony in the sterile broth. Always keep the burner on during inoculation and perform the action close to burner to avoid contamination. NOTE: the slides are very much similar to the IDD-1. Extraction of bacterial protein, IDD-41. Liquid chromatography - Gel filtration. Please do refer and perform animation accordingly.

1 Step 8: T 2: Preparation for ICAT 2 3 4 5 Audio Narration (if any) Description of the action Show a measuring balance, with display, ON, OFF and TARE/0 buttons on it. let user ON it, When measuing with paper, the weight display reading as 0. 000 g, let user picks up the of the paper need to be tared from paper from the rack, makes 1/10 of folding on actual reading. the sides and places it on the balance. Now the display reading changes to 0. 003 g. Instruct user to TARE the reading. And animate to click the tare button. Once user clicks it, reading must show ” 0”

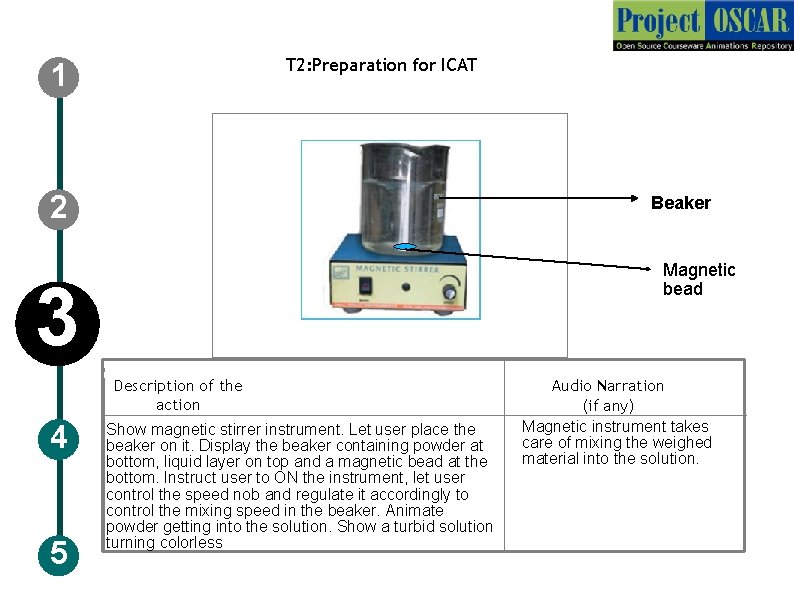
T 2: Preparation for ICAT 1 2 Beaker Magnetic bead 3 Description of the action 4 5 Show magnetic stirrer instrument. Let user place the beaker on it. Display the beaker containing powder at bottom, liquid layer on top and a magnetic bead at the bottom. Instruct user to ON the instrument, let user control the speed nob and regulate it accordingly to control the mixing speed in the beaker. Animate powder getting into the solution. Show a turbid solution turning colorless Audio Narration (if any) Magnetic instrument takes care of mixing the weighed material into the solution.

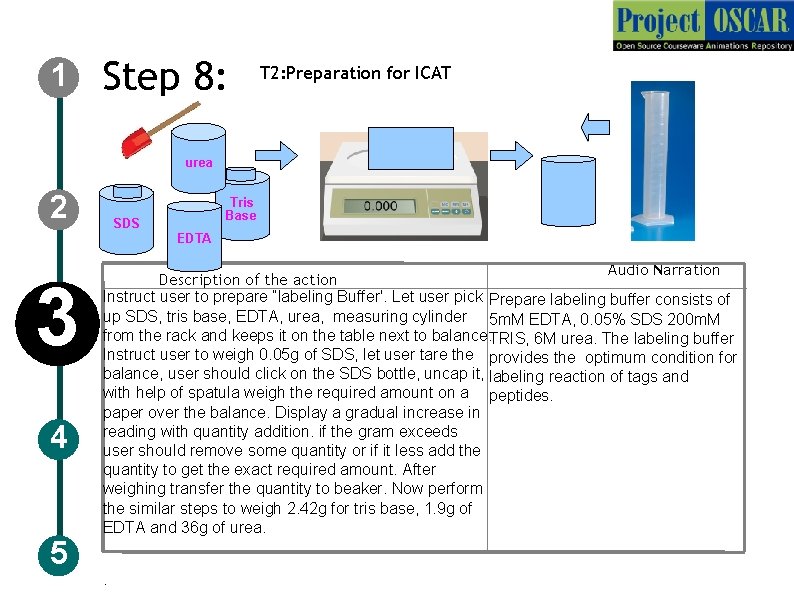
1 Step 8: T 2: Preparation for ICAT urea 2 Tris Base SDS EDTA 3 4 5 Audio Narration Description of the action Instruct user to prepare “labeling Buffer'. Let user pick Prepare labeling buffer consists of up SDS, tris base, EDTA, urea, measuring cylinder 5 m. M EDTA, 0. 05% SDS 200 m. M from the rack and keeps it on the table next to balance. TRIS, 6 M urea. The labeling buffer Instruct user to weigh 0. 05 g of SDS, let user tare the provides the optimum condition for balance, user should click on the SDS bottle, uncap it, labeling reaction of tags and with help of spatula weigh the required amount on a peptides. paper over the balance. Display a gradual increase in reading with quantity addition. if the gram exceeds user should remove some quantity or if it less add the quantity to get the exact required amount. After weighing transfer the quantity to beaker. Now perform the similar steps to weigh 2. 42 g for tris base, 1. 9 g of EDTA and 36 g of urea. .

1 2 3 4 5 Step 8: T 2: Preparation for ICAT Labeling buffer Water bottle Description of the action Now instruct the user to take water bottle, open the cap, take 100 ml measuring cylinder, measure 90 ml. Let user remove the excess water if level crosses 90 ml mark. Transfer it to beaker. Now take the beaker, shake it to make a proper mix as shown in slide 31. Animate the powder getting into the solution. Now set the p. H to 8. 5 by using p. H meter. Audio Narration labeling Buffer must be prepared at specific p. H for better binding efficiency.

1 Step 8: T 2: Preparation for ICAT 2 3 4 5 STD 1 Description of the action STD 2 Audio Narration Before the p. H reading, p. H Display standard p. H bottles and p. H instrument and deionized water, discard bottle placed on a table. Instruct instrument need to be calibrated with standards. user to caliberate the instrument. Let user ON the instrument. Initially for the p. H rod is dipped in water, when Once with STD 1 at p. H 4 and user clicks on read button, display must show a reading with STD 2 at p. H 9. “ 7”. Now show like taking out the rod and washing it with deionized-water, let user cleans the rod with tissue. Now pick the STD 1, uncap it, dip the cleaned rod into the solution, user must click read button with display showing “ 4”. now clean the rod and repeat the step to note down the reading for STD 2 and now the display should show “ 9”

1 Step 8: T 2: Preparation for ICAT 2 Na. OH HCl 3 4 5 Description of the action Instruct user to set the p. H for labeling buffer p. H at 8. 5. Now take the labeling buffer bottle, uncap it, dip the cleaned p. H rod into the solution. User need to click on read button. Initially display must show a reading 6. now instruct user to add Na. OH to adjust the p. H. Now allow the user to click on Na. OH bottle so that drops of Na. OH should be added with filler, user need to mix the solution with glass rod, click on read button and the reading should show anywhere near 6. 1 - 6. 3. let user keeps adding the Na. OH drop till the p. H display shows 8. 5 and later transfer the beaker solution to 100 ml measuring cylinder to makeup the volume to 100 ml by clicking on water and adding it to that. All action should happen when the user clicks the hand. Audio Narration Set the p. H of Labeling buffer to p. H 8. 5 by adding Na. OH drop by drop. In case of increase in p. H do balnce it by adding HCl.

1 Step 9: T 2: Preparation for ICAT 2 3 4 5 Audio Narration (if any) Description of the action Show a measuring balance, with display, ON, OFF and TARE/0 buttons on it. let user ON it, When measuing display reading as 0. 000 g, let user picks up the with paper, the paper from the rack, makes 1/10 of folding on the sides and places it on the balance. Now the weight of the paper need to be tarred display reading changes to 0. 003 g. Instruct user to TARE the reading. And animate to click from actual the tare button. Once user clicks it, reading. must show ” 0”

1 Step 9: T 2: Preparation for ICAT Guanidine hcl 2 Tributyl phosphine 3 4 5 Tris Base Audio Narration Description of the action Instruct user to prepare “Reducing solution buffer”. Let Prepare reducing solution buffer user pick up, tris base, guanidine HCl, tributyl consists of 5 m. M tributyl phosphine, measuring cylinder from the rack and 50 m. M TRIS, 6 M guanidine hcl. keeps it on the table next to balance. Instruct user to weigh 0. 06 g of Tris, let user tare the balance, user should click on the Tris bottle, uncap it, with help of spatula weigh the required amount on a paper over the balance. Display a gradual increase in reading with quantity addition. if the gram exceeds user should remove some quantity or if it less add the quantity to get the exact required amount. After weighing transfer the quantity to beaker. Now 5. 73 g accordingly for guanidine HCL , 0. 01 g of tributyl phosphine. Follow step as in slide: 32.

1 2 3 4 5 Step 9: T 2: Preparation for ICAT reducing Solution buffer water bottle Description of the action Now instruct the user to take water bottle, open the cap, take 50 ml measuring cylinder, measure 40 ml. Let user remove the excess water if level crosses 40 ml mark. Transfer it to beaker. Now take the beaker, shake it to make a proper mix as shown in slide 31. Animate the powder getting into the solution. Now set the p. H to 8. 5 by using p. H meter. Audio Narration . labeling Buffer must be prepared at specific p. H for better binding efficiency.

1 Step 9: T 2: Preparation for ICAT 2 3 4 5 STD 1 Description of the action Display standard p. H bottles and p. H instrument and deionized water, discard bottle placed on a table. Instruct user to caliberate the instrument. Let user ON the instrument. Initially for the p. H rod is dipped in water, when user clicks on read button, display must show a reading “ 7”. Now show like taking out the rod and washing it with deionized-water, let user cleans the rod with tissue. Now pick the STD 1 , uncap it, dip the cleaned rod into the solution, user must click read button with display showing “ 4”. now clean the rod and repeat the step to note down the reading for STD 2 and now the display should show “ 9” STD 2 Audio Narration Before the p. H reading, p. H instrument need to be calibrated with standards. Once with STD 1 at p. H 4 and with STD 2 at p. H 9.

1 Step 9: T 2: Preparation for ICAT 2 Na. OH HCl 3 4 5 Description of the action Instruct user to set the p. H for reduction buffer p. H at 8. 5. Now take the reduction buffer bottle, uncap it, dip the cleaned p. H rod into the solution. User need to click on read button. Initially display must show a reading 6. now instruct user to add Na. OH to adjust the p. H. Now allow the user to click on Na. OH bottle so that drops of Na. OH should be added with filler, user need to mix the solution with glass rod, click on read button and the reading should anywhere near 6. 1 - 6. 3. let user keeps adding the Na. OH drop till the p. H display shows 8. 5 and later transfer the beaker solution to 50 ml measuring cylinder to makeup the volume to 50 ml by clicking on water and adding it to that. All action should happen when the user clicks the hand Audio Narration Set the p. H of reduction buffer to p. H 8. 5 with Na. OH in case if the is more do add HCl to bring back to the required p. H.

1 Step 10: T 2: Preparation for ICAT Labeling buffer 2 3 4 5 Description of the action Instruct user to add “labeling buffer” to the tubes having the pellet. Now instruct the user to click on the pipette set to 300 ul and take the labeling solution into it when the user clicks and add to both the tubes. Show the vortex as in slide 27. Zoom in the tubes to show clear solution. Audio Narration Add labeling solution to the pellet to dissolve it completely by a small vortex.

1 Step 11: T 2: Preparation for ICAT reducing solution 2 3 4 Description of the action Now show the bottle labeled as “Reduction buffer solution”. Now instruct the user to click on the pipette set to 300 ul and take the reduction solution into it when the user clicks on it and add to both the tubes. Show the vortex as in slide 27. Zoom in the tubes to show clear solution. 5 Audio Narration Add reducing solution to the pellet followed by a small vortex. This helps for reducing the disulphide bonds in the protein.

1 Step 12: T 2: Preparation for ICAT 2 3 4 5 Description of the action Animate a instrument as in figure, Instruct the user to click on set and increase the temperature to 37 C and click start, Show the gradual increase in temperature and reaching 37 C after sometime. Now animate like the user taking the tubes and placing inside the instrument by opening it and animate a clock for 1 hr Audio Narration Place the sample at 37’C for 1 hr for reducing reaction.

1 Step 13: T 2: Preparation for ICAT Description of the action 2 3 4 5 Now Show like the user doing the quantification as in IDD: 11 protein quantification. Show the protein quantity as 150 ug in both the tubes. (control/drug treated) Audio Narration Estimate the protein quantity to have a rough idea to add the ICAT reagent. The quantity of protein required is around 150 ug for ideal ICAT experiment.

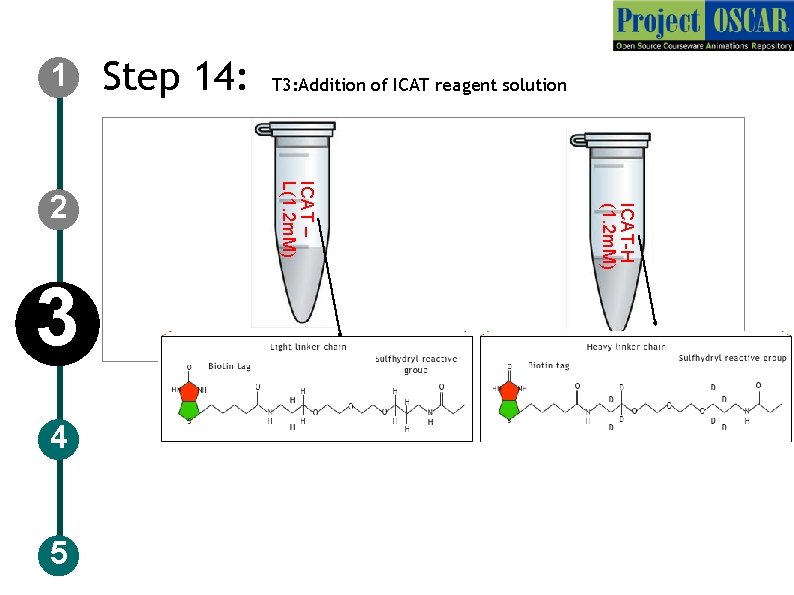
1 4 5 ICAT-H (1. 2 m. M) 3 T 3: Addition of ICAT reagent solution ICAT – L(1. 2 m. M) 2 Step 14:

1 Step 14: T 3: Addition of ICAT reagent solution Description of the action 2 3 4 5 Show the tubes as in slide 45 and the user should click on it to get the structure details followed by narration. Now instruct the user to click on pipette, set the pipette to 1000 ul and take the ICAT-L reagent (5 fold excess than protein) to add to the Control tube and in same way ICAT-H to add to the “Drug treated”. Animate like the user placing it in the incubator(fig in slide 43) at 37’C for 1 hr animate a clock. After 1 hr animate let the user take out the sample tubes, set the pipette to 100 ul and take 100 ul volume of each sample and add to the empty tube labeled as “mixed sample” Audio Narration Add 5 fold excess of ICAT reagents with light labeled to control sample and heavy labeled isotope to the drug treated and place it in incubator at 37’C for 1 hr and mix the sample in 1: 1 ratio. ICAT reagents bind to the cysteine residues in the protein sample. The sample is ready for gel filtration.

1 Step 15: T 4: Gel filtration 2 3 4 5 Audio Narration (if any) Description of the action Show a measuring balance, with display, ON, OFF and TARE/0 buttons on it. let user ON it, When measuring with paper, the display reading as 0. 000 g, let user picks up the weight of the paper need to be tarred paper from the rack, makes 1/10 of folding on the sides and places it on the balance. Now the from actual reading. display reading changes to 0. 003 g. Instruct user to TARE the reading. And animate to click the tare button. Once user clicks it, reading must show ” 0”

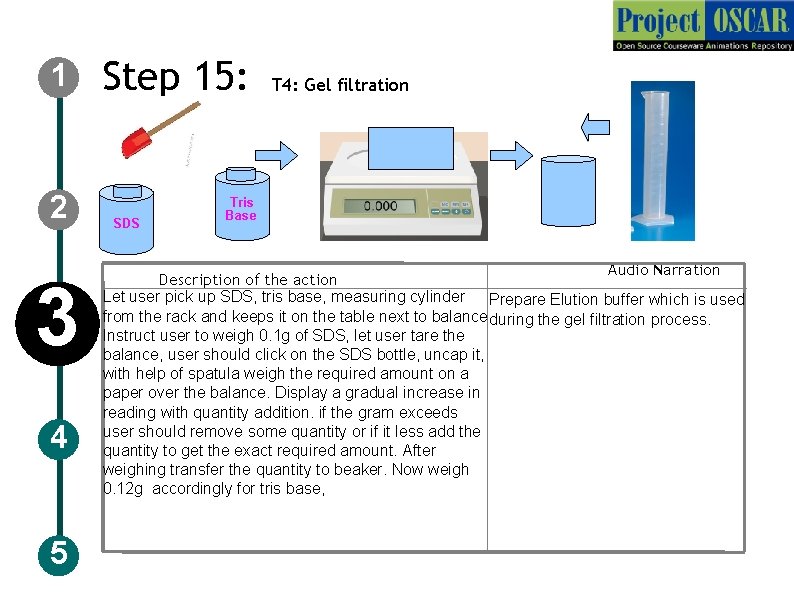
1 2 3 4 5 Step 15: SDS T 4: Gel filtration Tris Base Audio Narration Description of the action Let user pick up SDS, tris base, measuring cylinder Prepare Elution buffer which is used from the rack and keeps it on the table next to balance. during the gel filtration process. Instruct user to weigh 0. 1 g of SDS, let user tare the balance, user should click on the SDS bottle, uncap it, with help of spatula weigh the required amount on a paper over the balance. Display a gradual increase in reading with quantity addition. if the gram exceeds user should remove some quantity or if it less add the quantity to get the exact required amount. After weighing transfer the quantity to beaker. Now weigh 0. 12 g accordingly for tris base,

1 Step 15: T 4: Gel filtration 2 3 4 5 Description of the action Now instruct the user to take water bottle, open the cap, take 100 ml measuring cylinder, measure 90 ml. Let user remove the excess water if level crosses 90 ml mark. Transfer it to beaker containing the weighed SDS and Tris. Base from previos slide. Now take the beaker, place it on magnetic bead rotar for a proper mix as shown in slide: 30. Animate the powder getting into the solution. Now set the p. H to 8. 5 by using p. H meter. Audio Narration

1 Step 15: T 4: Gel filtration 2 3 4 5 STD 1 Description of the action STD 2 Audio Narration Before the p. H reading, p. H Display standard p. H bottles and p. H instrument and deionized water, discard placed on a table. Instruct user to instrument need to be calibrated with standards. caliberate the instrument. Let user ON the instrument. Initially for the p. H rod is dipped in water, when user clicks Once with STD 1 at p. H 4 and on read button, display must show a reading “ 7”. Now with STD 2 at p. H 9. show like taking out the rod and washing it with deionized -water, let user cleans the rod with tissue. Now pick the STD 1 , uncap it, dip the cleaned rod into the solution, user must click read button with display showing “ 4”. now clean the rod and repeat the step to note down the reading for STD 2 and now the display should show “ 9”

1 Step 15: T 4: Gel filtration 2 3 4 5 Na. OH HCl Description of the action Instruct user to set the p. H for labeling buffer p. H at 8. 5. Now take the Elution buffer bottle, uncap it, dip the cleaned p. H rod into the solution. User need to click on read button. Initially display must show a reading 6. now instruct user to add Na. OH to adjust the p. H. Now allow the user to click on Na. OH bottle so that drops of Na. OH should be added with filler, user need to mix the solution with glass rod, click on read button and the reading should anywhere near 6. 1 - 6. 3. let user keeps adding the Na. OH drop till the p. H display shows 8. 5 and later transfer the beaker solution to 100 ml measuring cylinder to makeup the volume to 100 ml by clicking on water and adding it to that. All action should happen when the user clicks the hand. Audio Narration Set the p. H of elution buffer to p. H 8. 5.

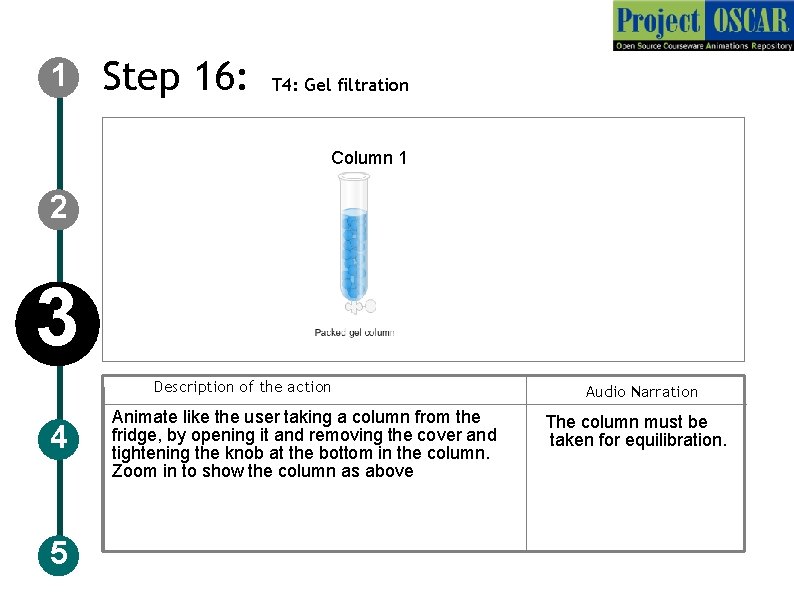
1 Step 16: T 4: Gel filtration AE DE Coulmn Column 1 2 3 Description of the action 4 5 Animate like the user taking a column from the fridge, by opening it and removing the cover and tightening the knob at the bottom in the column. Zoom in to show the column as above Audio Narration The column must be taken for equilibration.

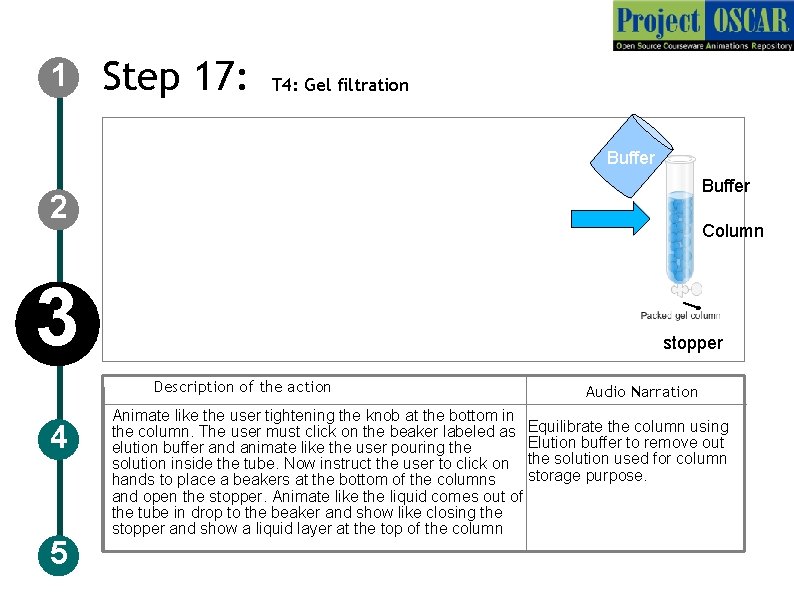
1 Step 17: T 4: Gel filtration Buffer 2 Column 3 stopper Description of the action 4 5 Audio Narration Animate like the user tightening the knob at the bottom in the column. The user must click on the beaker labeled as Equilibrate the column using Elution buffer to remove out elution buffer and animate like the user pouring the solution inside the tube. Now instruct the user to click on the solution used for column storage purpose. hands to place a beakers at the bottom of the columns and open the stopper. Animate like the liquid comes out of the tube in drop to the beaker and show like closing the stopper and show a liquid layer at the top of the column

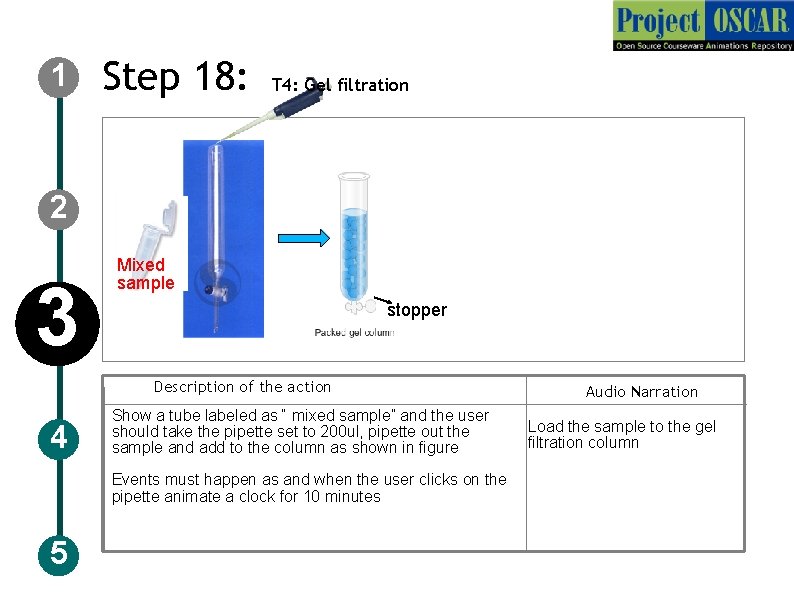
1 Step 18: T 4: Gel filtration 2 3 Mixed sample stopper Description of the action 4 Show a tube labeled as “ mixed sample” and the user should take the pipette set to 200 ul, pipette out the sample and add to the column as shown in figure Events must happen as and when the user clicks on the pipette animate a clock for 10 minutes 5 Audio Narration Load the sample to the gel filtration column

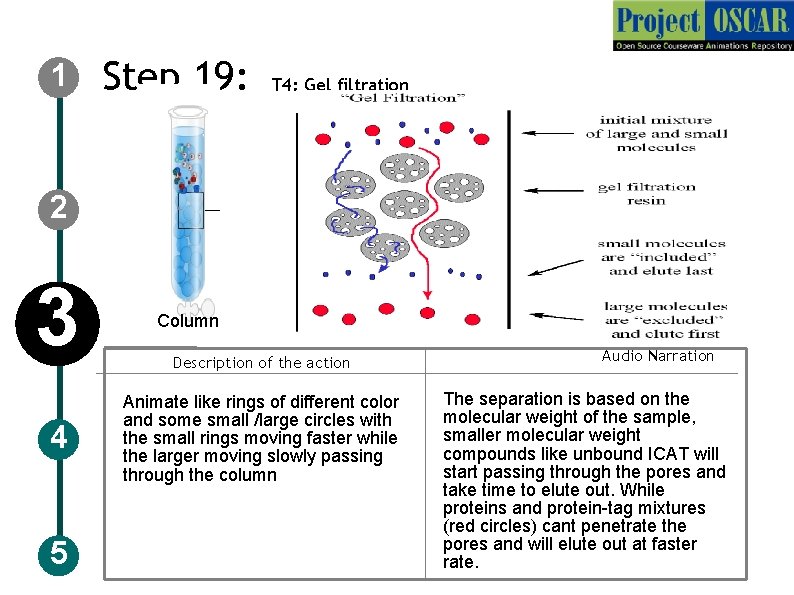
1 Step 19: T 4: Gel filtration 2 3 4 5 Column Description of the action Animate like rings of different color and some small /large circles with the small rings moving faster while the larger moving slowly passing through the column Audio Narration The separation is based on the molecular weight of the sample, smaller molecular weight compounds like unbound ICAT will start passing through the pores and take time to elute out. While proteins and protein-tag mixtures (red circles) cant penetrate the pores and will elute out at faster rate.

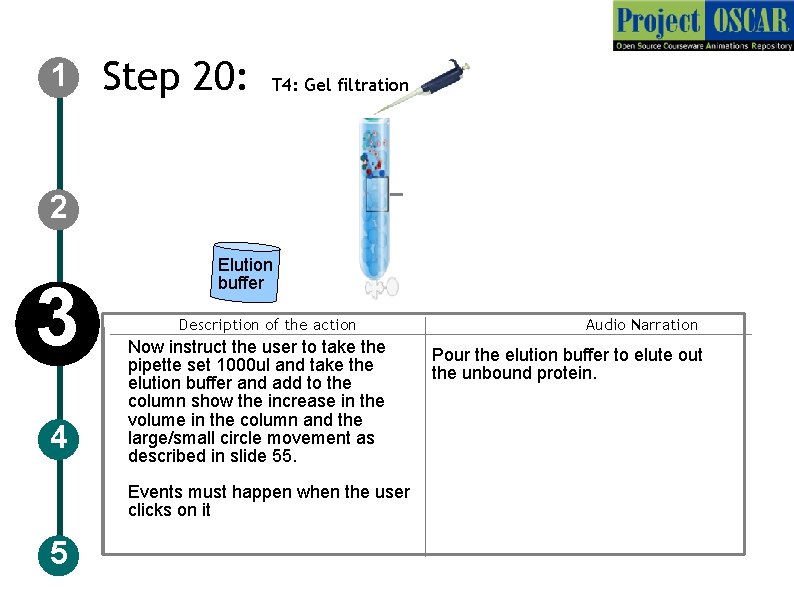
1 Step 20: T 4: Gel filtration 2 3 4 Elution buffer Description of the action Now instruct the user to take the pipette set 1000 ul and take the elution buffer and add to the column show the increase in the volume in the column and the large/small circle movement as described in slide 55. Events must happen when the user clicks on it 5 Audio Narration Pour the elution buffer to elute out the unbound protein.

1 2 3 4 5 Step 21: T 4: Gel filtration

1 2 3 4 5 Step 21: T 4: Gel filtration Description of the action From the slide 56, show the collection tubes in row and the solution form the column dropping into it. Show the tube 1 with elution solution, tube 2 with some larger rings and followed with other tubes. user should click on it and a tab should appear labeled as ”ICAT labeled proteins+ other proteins” increased amount of smaller rings in tube 3, 4 and less of smaller rings in tube 5, 6 and 7 and in tube 8 a tab should appear labeled as ”excess ICAT” and only solution in tube 9. Audio Narration In gel filtration, depending upon the pore size the separation takes place. For more information please follow IDD 41. Liquid chromatography - Gel filtration.

1 Step 22: T 5: In solution digestion 2 3 4 5 Description of the action/ interactivity The collected solution is taken for trypsin digestion. Animate like instructing the user to go through the IDD-28. In solution digestion. Animate the IDD-28. In solution digestion in the same labeled tubes. Audio Narration (if any) Go through IDD-28. In solution digestion techniques for trypsin digestion. Further separation step is carried out either by IDD-42. Liquid chromatography - affinity chromatography or by IDD-17. SDSPAGE.

1 Step 23: T 6: Affinity chromatography AE DE Coulmn AVIDINColumn 2 3 stopper Description of the action 4 5 Instruct user to carry out “Affinity separation” Animate like the user taking a column from the fridge, by opening it and removing the cover and tightening the knob at the bottom in the column. Zoom in to show the column as above. Audio Narration Take the avidin column from the fridge prepare for the wash.

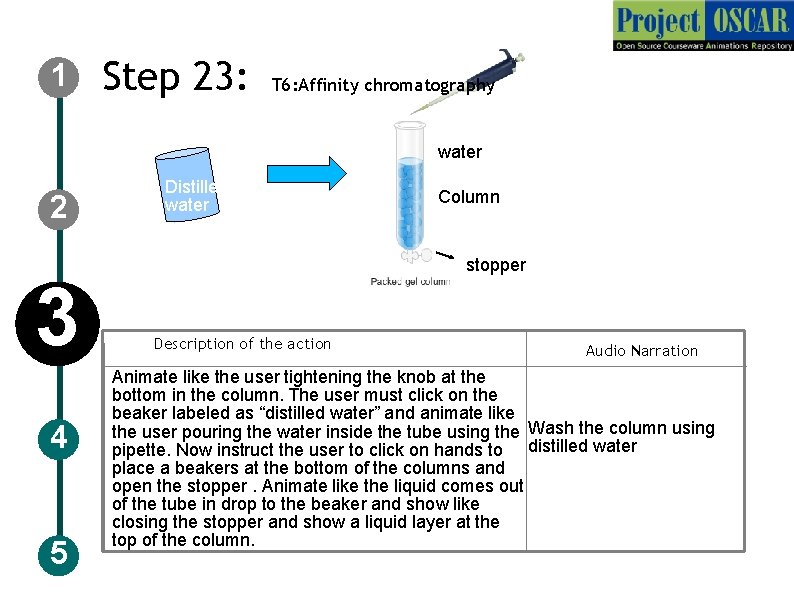
1 Step 23: T 6: Affinity chromatography water 2 3 4 5 Distilled water Column stopper Description of the action Audio Narration Animate like the user tightening the knob at the bottom in the column. The user must click on the beaker labeled as “distilled water” and animate like the user pouring the water inside the tube using the Wash the column using pipette. Now instruct the user to click on hands to distilled water place a beakers at the bottom of the columns and open the stopper. Animate like the liquid comes out of the tube in drop to the beaker and show like closing the stopper and show a liquid layer at the top of the column.

1 Step 23: T 6: Affinity chromatography 2 3 sample Description of the action 4 5 Audio Narration After In-solution digestion instruct user to carry out Load the sample to the affinity separation. Show a tube labeled as “tube avidin column for protein 5, 6” and the user should take the pipette set to 100 ul, pipette out the sample and add to the column separation. as shown in figure. Repeat the step for all the samples and add in the column for separation. Events must happen as and when the user clicks on the pipette animate a clock for 10 minutes

1 Step 24: T 6: Affinity chromatography 2 3 4 5 Column Description of the action Animate like rings of different color labeled as L and similarly other rings of different colors labeled as H show like the L and H binding to the column while rings without H or L labeling comes out Audio Narration The separation is based on the affinity of the biotin in the tag to the avidin in the column

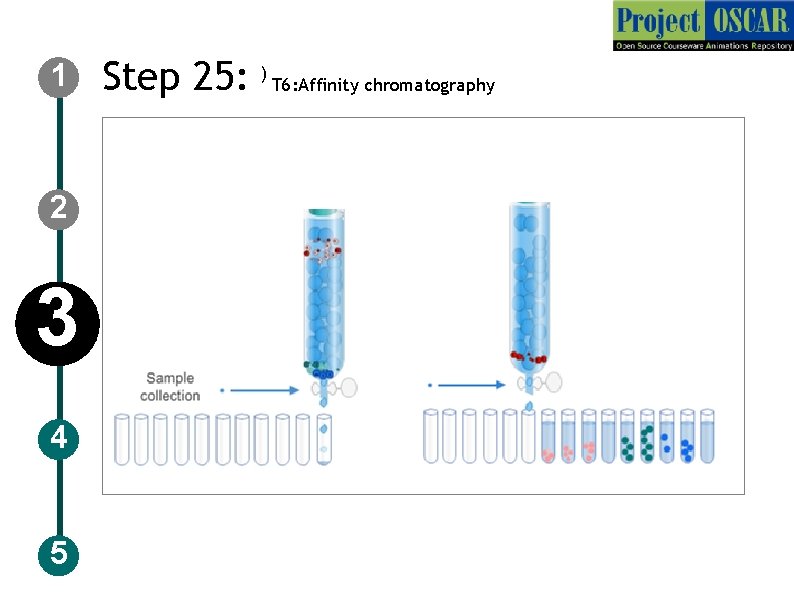
1 Step 25: T 6: Affinity chromatography 2 3 4 5 Description of the action Now instruct the user to take the pipette set 1000 ul and take bottle labeled as 0. 1% formic acid and add to the column show the increase in the volume in the column and the circles with different labelings movement as described in slide 57. Events must happen when the user clicks on it Audio Narration Pour 0. 1% formic acid containing 0. 1 ml formic acid and 99. 9 ml water to the column

1 2 3 4 5 Step 25: ) T 6: Affinity chromatography

1 2 3 4 5 Step 25: T 6: Affinity chromatography Description of the action Show the tubes in row and the solution dropping into it. Show the tube 1 only solution tube 2 with unlabeled rings, user should click on it and a tab should appear labeled as ”unlabeled proteins” and tube 3 with more of unlabeled rings tube 4 with some labeled rings user should click on it and a tab should appear labeled as”Isotope tagged proteins” and increased amount of L and H rings in tube 5, 6 and less of L and H rings in tube 7, 8 and only solution in tube 9. Instruct the user to take the tube 5, 6, 7, 8 for analysis Enriched isotope labeled peptides were taken for IDD-39. LC-MSMS data analysis

1 2 3 4 5 Step 26: T 7: LC/MS/MS and Spectrum analysis Instruct the user to go through future viewing IDDs for detailed information

Slide 5 Tab 01 Slide 6 -20 Tab 02 Slide 20 -22 Tab 03 Slide 23 -34 Tab 04 Slide 35 Slide 36 -42 Slide 43 Tab 06 Tab 07 Tab 05 Name of the section/stage Animation area INTERACTION 1: slide-46: user goes for 1: 1(instead of 1: 5) labeling and proceeds further. Instruction: In MS spectrum, depict very low intensity peaks (from IDD LC-MS/MS) and instruct user to go back and check the labeling step to make sure the ratio is 1: 5. Interactivity area Button 01 Button 02 Button 03 Instructions/ Working area Credits

1 Questionnaire 1. Which of the following amino acid residues is modified during ICAT analysis? Answers: a) Aspartic acid b) Lysine c) Cysteine d) Proline 2. Which chromatographic technique is employed to purify the labeled peptide fragments? 2 Answers: a) Strong cation exchange b) Gel filtration c) Hydrophobic interaction chromatography d) Affinity (avidin –Biotin )chromatography 3. Excess ICAT is removed by 3 Answers: a) Strong cation exchange b) Gel filtration c) Hydrophobic interaction chromatography d) Affinity (avidin –Biotin )chromatography 4. Biotin –avidin bound proteins are removed using a)Formic acid b) acetic acid c) ethanol d) DTT 4 5 5) If the concentration of protein is 5 ug/ul what will be the quantity of ICAT to be added to have efficient labling a)45 ug/ul b) 50 ug/ul c)25 ug/ul d) 24 ug/ul

APPENDIX 2 Links for further reading 1. 2. • • Reference websites: http: //www. proteomecenter. org/protocols/Acid%20 Cle avable%20 ICAT%20 Labelling%20 Protocol. pdf http: //www. bio. davidson. edu/courses/genomics/ICAT/ ICAT. html Research papers: Gygi, S. P. et al. , Quantitative analysis of complex protein mixtures using isotope-coded affinity tags. Nat Biotech. 1999, 17: 994 -999.

APPENDIX 3 Summary The experiment mainly focuses on the cell culturing, protein extraction, ICAT labeling, gel filtration followed by in solution digestion , affinity chromatography , LC/MS/MS and LC/MS/MS analysis. These technique is used to detect the proteins that show variable expression in the control /treated or diseased one.