Isotope chronology of meteorites and oxygen isotopes Part


- Slides: 12

Isotope chronology of meteorites and oxygen isotopes Part I: Radiometric dating methods Esa Vilenius 13. 2. 2006 Outline • Introduction • Rubidium-Strontium chronometer • Problems of radiometric chronometers • Lead-lead method • Short-lived isotopes • Chronology of early Solar System

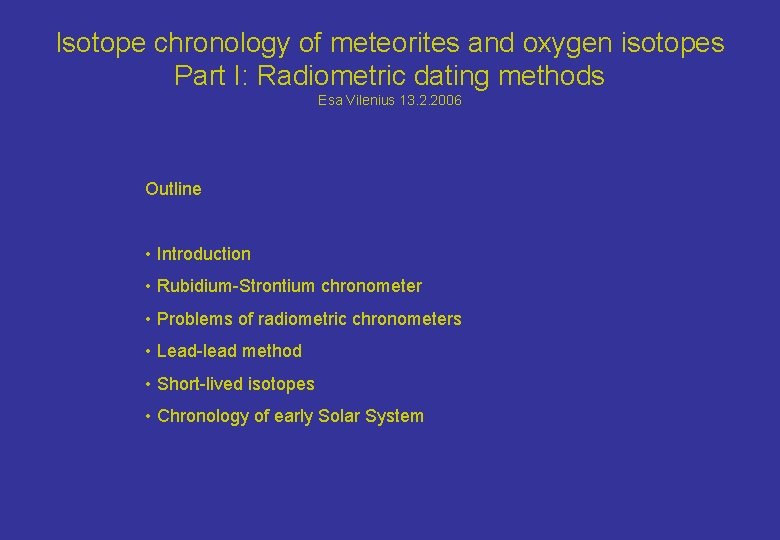
What can be dated? - Formation age of solid material - Formation intervals (relative to other meteorites) - Reheating events (metamorphic ages) - Cosmic ray exposure age (meter-sized objects) - Terrestrial age

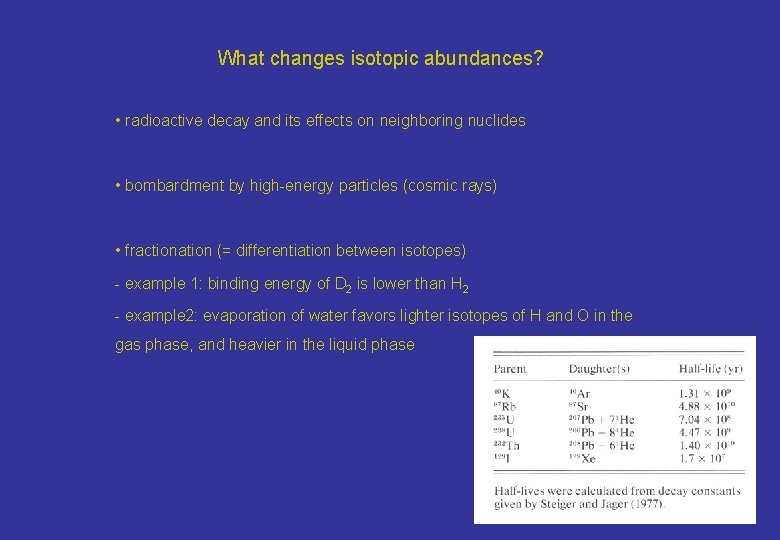
What changes isotopic abundances? • radioactive decay and its effects on neighboring nuclides • bombardment by high-energy particles (cosmic rays) • fractionation (= differentiation between isotopes) - example 1: binding energy of D 2 is lower than H 2 - example 2: evaporation of water favors lighter isotopes of H and O in the gas phase, and heavier in the liquid phase

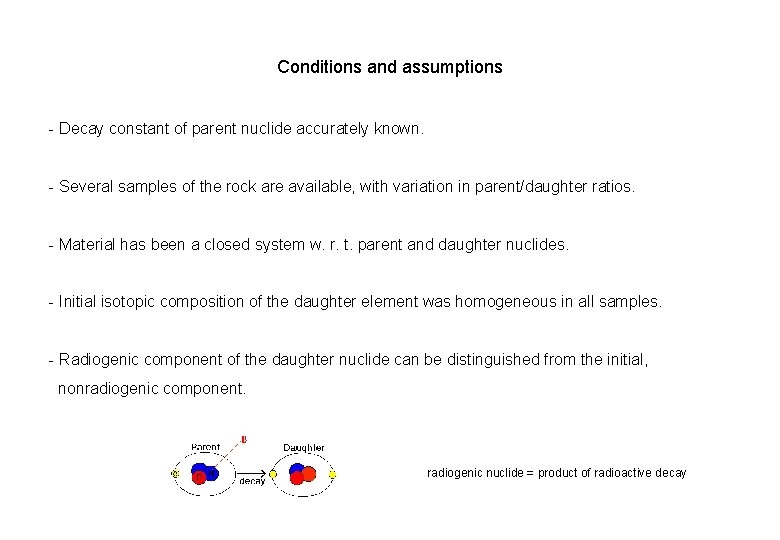
Conditions and assumptions - Decay constant of parent nuclide accurately known. - Several samples of the rock are available, with variation in parent/daughter ratios. - Material has been a closed system w. r. t. parent and daughter nuclides. - Initial isotopic composition of the daughter element was homogeneous in all samples. - Radiogenic component of the daughter nuclide can be distinguished from the initial, nonradiogenic component. radiogenic nuclide = product of radioactive decay

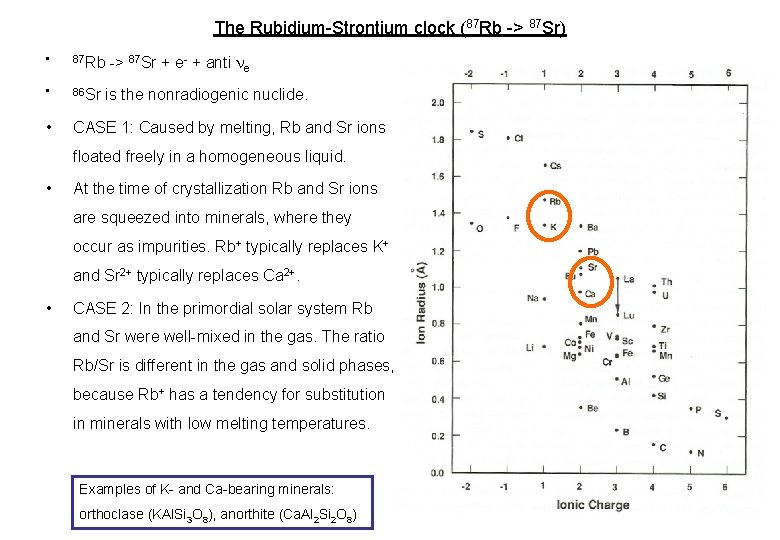
The Rubidium-Strontium clock (87 Rb -> 87 Sr) -> 87 Sr + e- + anti ne • 87 Rb • 86 Sr • CASE 1: Caused by melting, Rb and Sr ions is the nonradiogenic nuclide. floated freely in a homogeneous liquid. • At the time of crystallization Rb and Sr ions are squeezed into minerals, where they occur as impurities. Rb+ typically replaces K+ and Sr 2+ typically replaces Ca 2+. • CASE 2: In the primordial solar system Rb and Sr were well-mixed in the gas. The ratio Rb/Sr is different in the gas and solid phases, because Rb+ has a tendency for substitution in minerals with low melting temperatures. Examples of K- and Ca-bearing minerals: orthoclase (KAl. Si 3 O 8), anorthite (Ca. Al 2 Si 2 O 8)

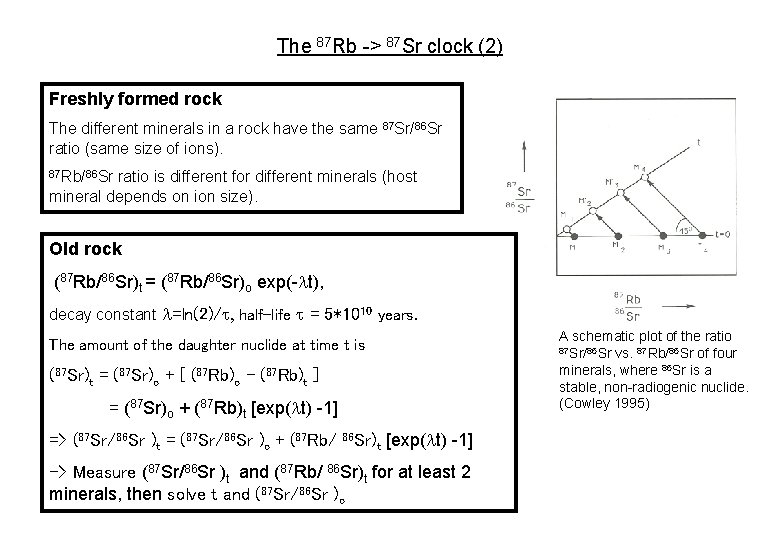
The 87 Rb -> 87 Sr clock (2) Freshly formed rock The different minerals in a rock have the same 87 Sr/86 Sr ratio (same size of ions). 87 Rb/86 Sr ratio is different for different minerals (host mineral depends on ion size). Old rock (87 Rb/86 Sr)t = (87 Rb/86 Sr)o exp(-lt), decay constant l=ln(2)/t, half-life t = 5*1010 years. The amount of the daughter nuclide at time t is (87 Sr)t = (87 Sr)o + [ (87 Rb)o - (87 Rb)t ] = (87 Sr)o + (87 Rb)t [exp(lt) -1] => (87 Sr/86 Sr )t = (87 Sr/86 Sr )o + (87 Rb/ 86 Sr)t [exp(lt) -1] -> Measure (87 Sr/86 Sr )t and (87 Rb/ 86 Sr)t for at least 2 minerals, then solve t and (87 Sr/86 Sr )o A schematic plot of the ratio 87 Sr/86 Sr vs. 87 Rb/86 Sr of four minerals, where 86 Sr is a stable, non-radiogenic nuclide. (Cowley 1995)

The 87 Rb -> 87 Sr clock (3) Example of results 1: H-group chondrites Whole-rock Rb-Sr isochron of 16 H-chondrite meteorites => Common formation age 4. 69± 0. 07 Gyr. Example of results 2: formation intervals Initial 87 Sr/86 Sr ratios from isochrons of 6 meteorites. Kaushal and Wetherill (1969)

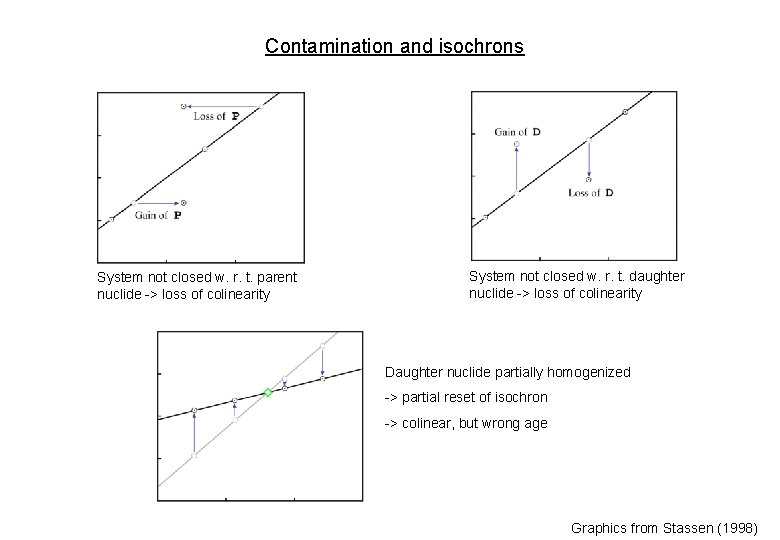
Contamination and isochrons System not closed w. r. t. parent nuclide -> loss of colinearity System not closed w. r. t. daughter nuclide -> loss of colinearity Daughter nuclide partially homogenized -> partial reset of isochron -> colinear, but wrong age Graphics from Stassen (1998)

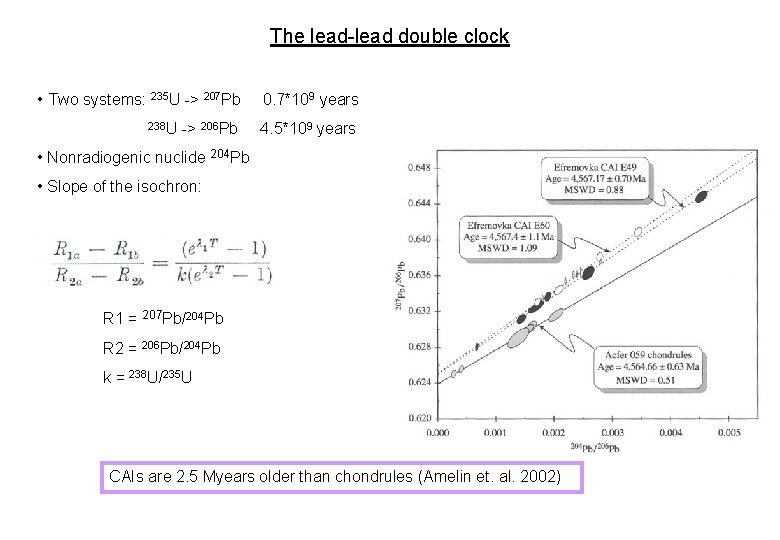
The lead-lead double clock • Two systems: 235 U -> 207 Pb 238 U -> 206 Pb 0. 7*109 years 4. 5*109 years • Nonradiogenic nuclide 204 Pb • Slope of the isochron: R 1 = 207 Pb/204 Pb R 2 = 206 Pb/204 Pb k = 238 U/235 U CAIs are 2. 5 Myears older than chondrules (Amelin et. al. 2002)

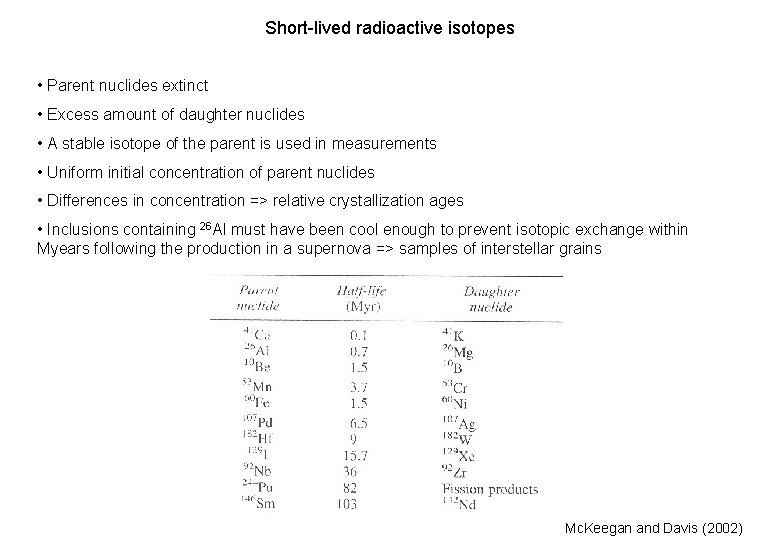
Short-lived radioactive isotopes • Parent nuclides extinct • Excess amount of daughter nuclides • A stable isotope of the parent is used in measurements • Uniform initial concentration of parent nuclides • Differences in concentration => relative crystallization ages • Inclusions containing 26 Al must have been cool enough to prevent isotopic exchange within Myears following the production in a supernova => samples of interstellar grains Mc. Keegan and Davis (2002)

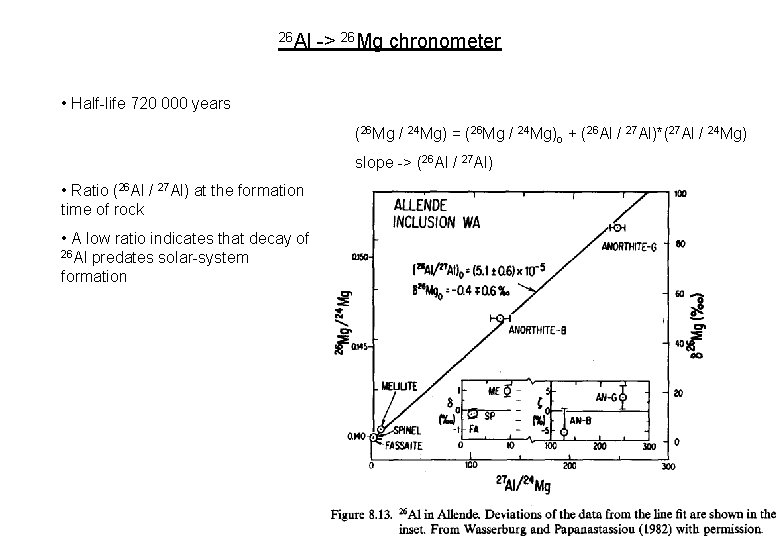
26 Al -> 26 Mg chronometer • Half-life 720 000 years (26 Mg / 24 Mg) = (26 Mg / 24 Mg)o + (26 Al / 27 Al)*(27 Al / 24 Mg) slope -> (26 Al / 27 Al) • Ratio (26 Al / 27 Al) at the formation time of rock • A low ratio indicates that decay of 26 Al predates solar-system formation

Early Solar System chronology • At 4568 Ma a supernova triggers gravitational collapse. • • CAIs are the first solid material (aluminium-26 relative ages) Formation of CAIs 4567. 2 ± 0. 6 Ma (lead-lead isochron). • • Formation of chondrules 4564. 7 ± 0. 6 Ma (lead-lead isochron), lasting 1 -2 Myears. • • CAIs join chondrules forming chondrites at 4565 - 4564 Myears, melting and differentiation of meteorite parent bodies. www. spacedaily. com Allende CV 3, 200 x zoom www. zeiss. com www. spaceflightnow. com