Isotonicity PHT 434 osmosis Osmosis is the diffusion








- Slides: 21

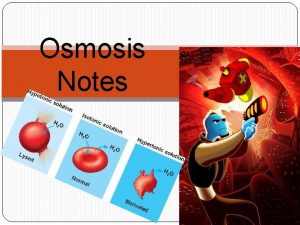
Isotonicity PHT 434

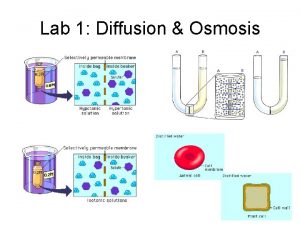
osmosis • Osmosis is the diffusion of solvent through a semipermeable membrane. ▫ Water always flows from lower solute concentration [dilute solution] to higher solute concentration until a balance is produced • Osmotic pressure is the force that cause this diffusion. • Tonicity is a measure of the osmotic pressure of two solutions separated by a semi-permeable membrane.

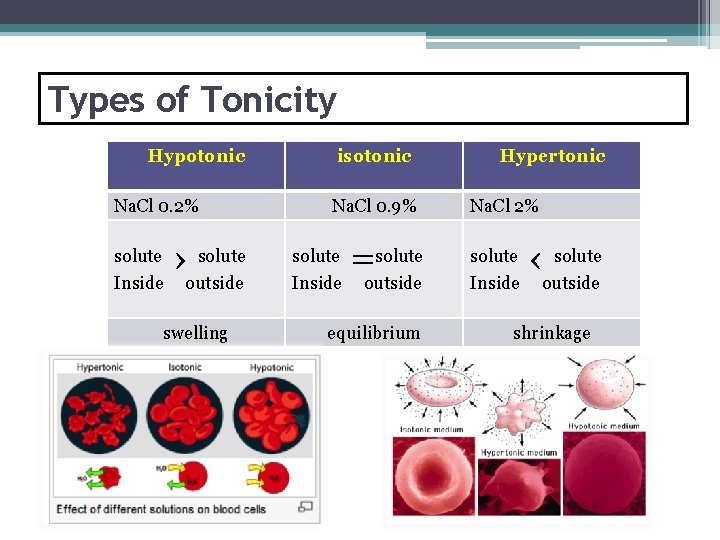
Types of Tonicity Hypotonic Na. Cl 0. 2% solute Inside solute ›outside swelling isotonic Na. Cl 0. 9% solute Inside solute =outside equilibrium Hypertonic Na. Cl 2% solute Inside solute ‹outside shrinkage

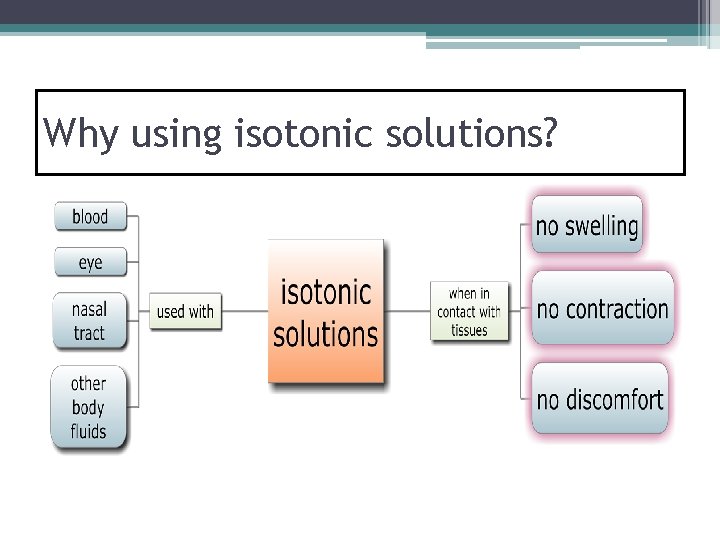
Why using isotonic solutions?

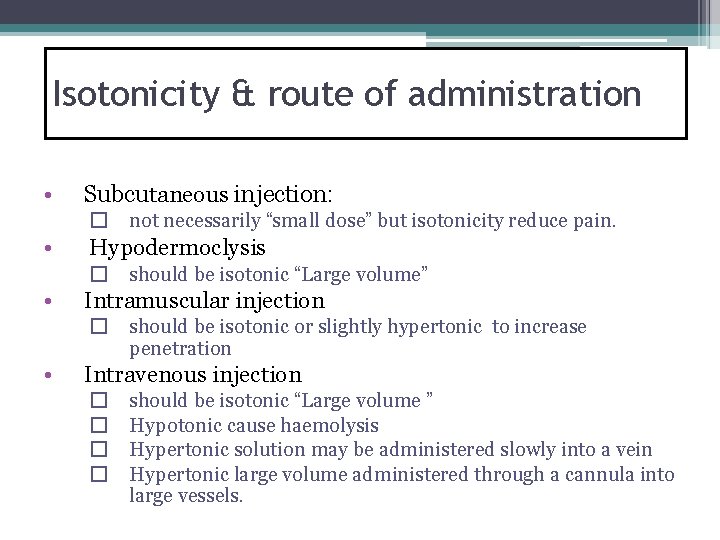
Isotonicity & route of administration • Subcutaneous injection: � not necessarily “small dose” but isotonicity reduce pain. • Hypodermoclysis � should be isotonic “Large volume” • Intramuscular injection � should be isotonic or slightly hypertonic to increase penetration • Intravenous injection � � should be isotonic “Large volume ” Hypotonic cause haemolysis Hypertonic solution may be administered slowly into a vein Hypertonic large volume administered through a cannula into large vessels.

Isotonicity & route of administration • cont. Intrathecal injestion � Should be isotonic • Eye drops � Rapid diluted by tear, but most of it is isotonic to decrease irritation • Eye lotions � Preferably isotonic • Nasal drops � Isotonic, but not essentially

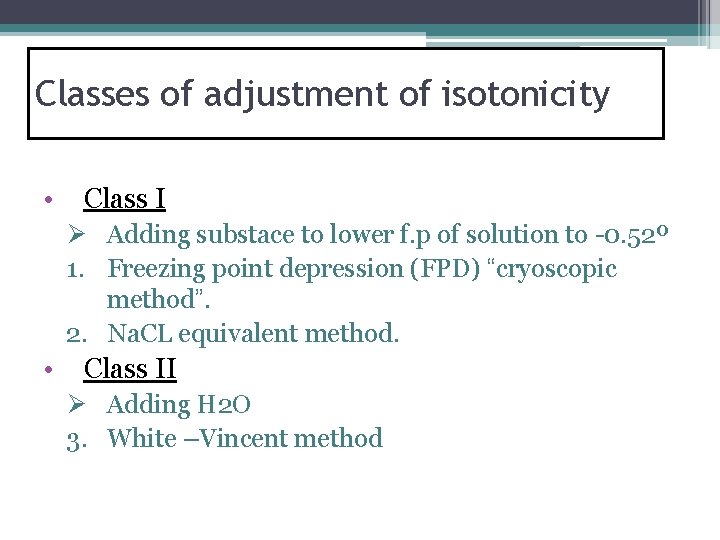
Classes of adjustment of isotonicity • Class I Ø Adding substace to lower f. p of solution to -0. 52º 1. Freezing point depression (FPD) “cryoscopic method”. 2. Na. CL equivalent method. • Class II Ø Adding H 2 O 3. White –Vincent method

Freezing point depression (f. p. d) • Freezing Pointsolution = Freezing Pointsolvent - ΔTf • ΔTf =L c L : constant , c : conc. (molarity) • It is Colligative property ▫ Depend on concetration ▫ same f. p. d same conc. same tonicity • 0. 9% Na. Cl is isotonic i. e. F. p. d = 0. 52º

1 - Freezing point depression (FPD) “cryoscopic method”. • F. P. of blood & tears = - 0. 52º • Any solution have F. P. = - 0. 52º is isotonic. • • Any solution have F. P. › - 0. 52º is hypotonic - 0. 4º hypotonic -0. 6º hypertonic Add solute to hypotonic solution to reach f. p. d of blood (- 0. 52º )

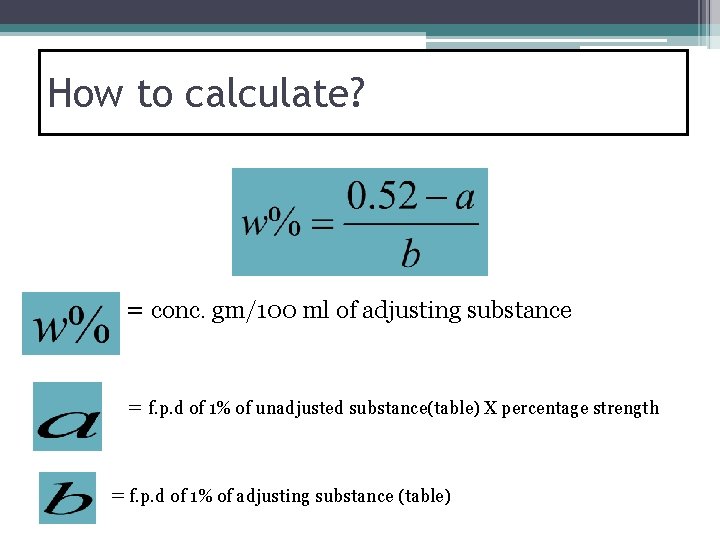
How to calculate? = conc. gm/100 ml of adjusting substance = f. p. d of 1% of unadjusted substance(table) X percentage strength = f. p. d of 1% of adjusting substance (table)

Example I • How much Na. Cl is required to render 100 ml of a 1% soln. of apomorphin HCL isotonic? • F. p. d of 1%Na. Cl=0. 58º, F. p. d of 1%drug=0. 08º • 1% drug • 1% Na. Cl w% Na. Cl 0. 08º (0. 52º- 0. 08º=0. 44º) 0. 58º 0. 44º

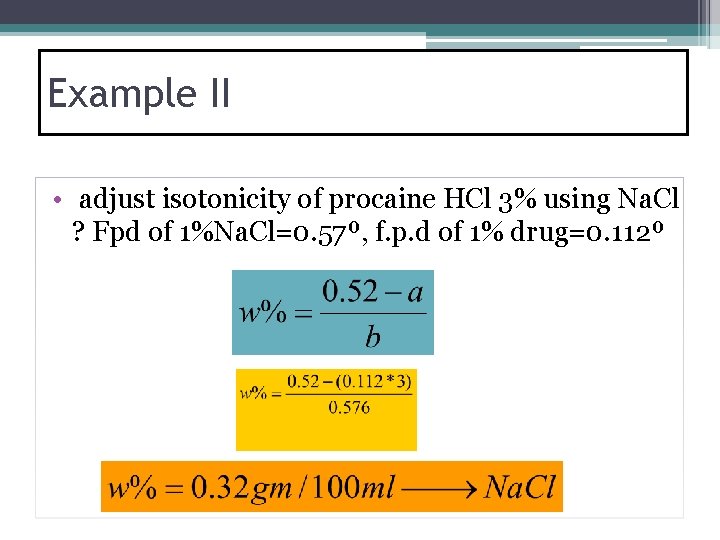
Example II • adjust isotonicity of procaine HCl 3% using Na. Cl ? Fpd of 1%Na. Cl=0. 57º, f. p. d of 1% drug=0. 112º

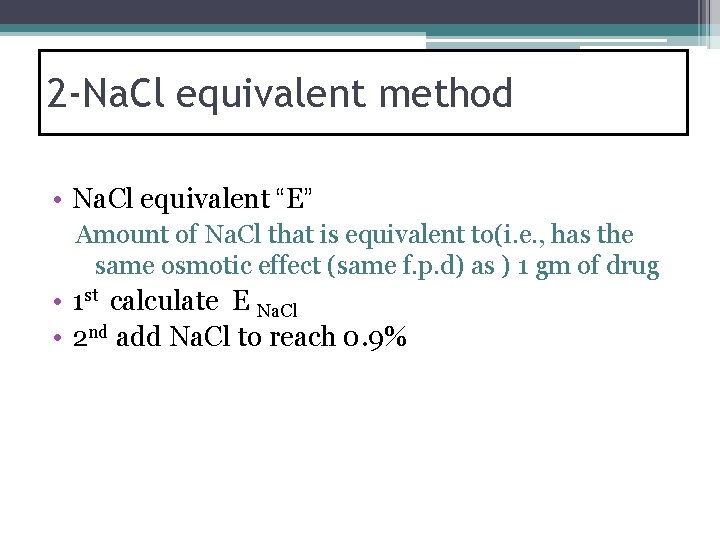
2 -Na. Cl equivalent method • Na. Cl equivalent “E” Amount of Na. Cl that is equivalent to(i. e. , has the same osmotic effect (same f. p. d) as ) 1 gm of drug • 1 st calculate E Na. Cl • 2 nd add Na. Cl to reach 0. 9%

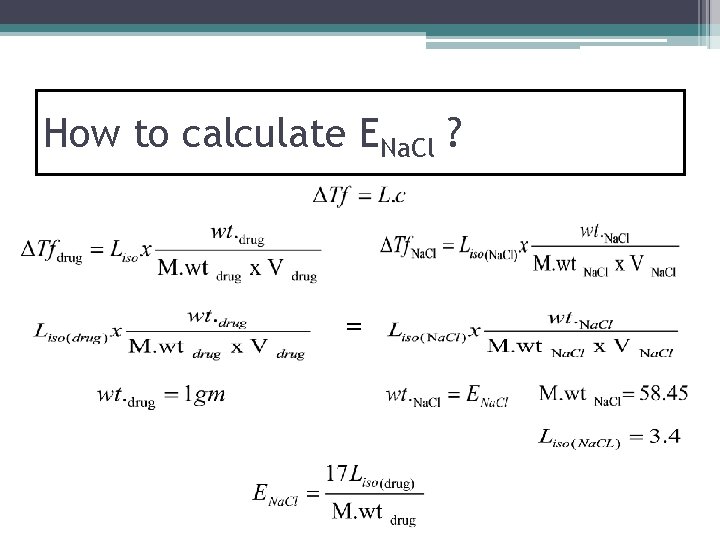
How to calculate ENa. Cl ? =

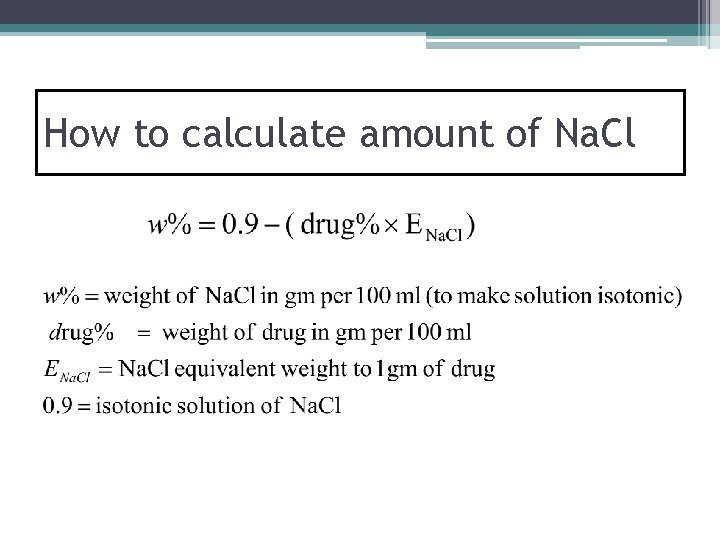
How to calculate amount of Na. Cl

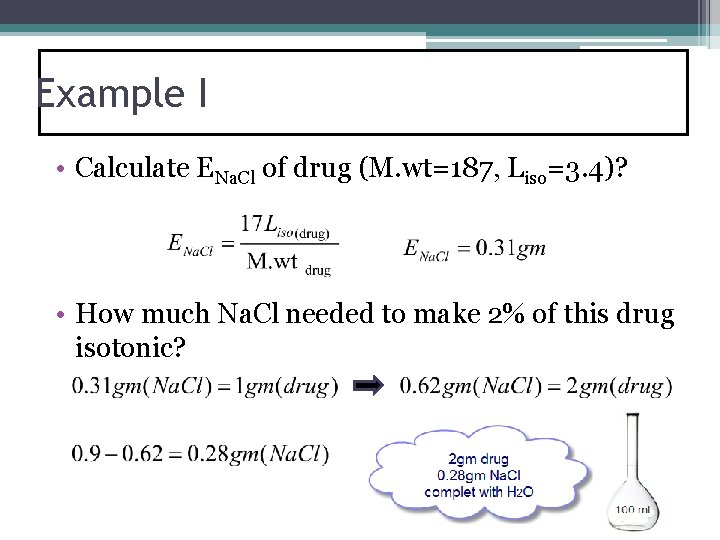
Example I • Calculate ENa. Cl of drug (M. wt=187, Liso=3. 4)? • How much Na. Cl needed to make 2% of this drug isotonic?

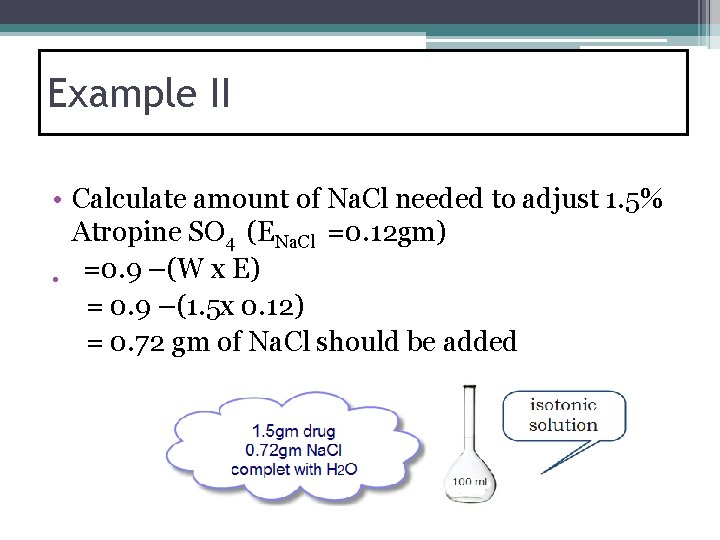
Example II • Calculate amount of Na. Cl needed to adjust 1. 5% Atropine SO 4 (ENa. Cl =0. 12 gm) • =0. 9 –(W x E) = 0. 9 –(1. 5 x 0. 12) = 0. 72 gm of Na. Cl should be added

3 -White – Vincent method • Principle: ▫ 1 st Addition of H 2 O to drug to make it isotonic ▫ 2 nd addition of isotonic vehicle to bring solution to final volume

How to calculate amount of H 2 O ? • Suppose preparing 30 ml of 1% drug isotonic with body fluid(ENa. Cl =0. 16 gm) • 1 gm 100 ml ? 30 ml =0. 3 gm • Amount of Na. Cl eq. to 0. 3 drug = 0. 3 x 0. 16 =0. 048 gm • 0. 9 gm 100 ml • 0. 048 gm ? ml =5. 3 ml

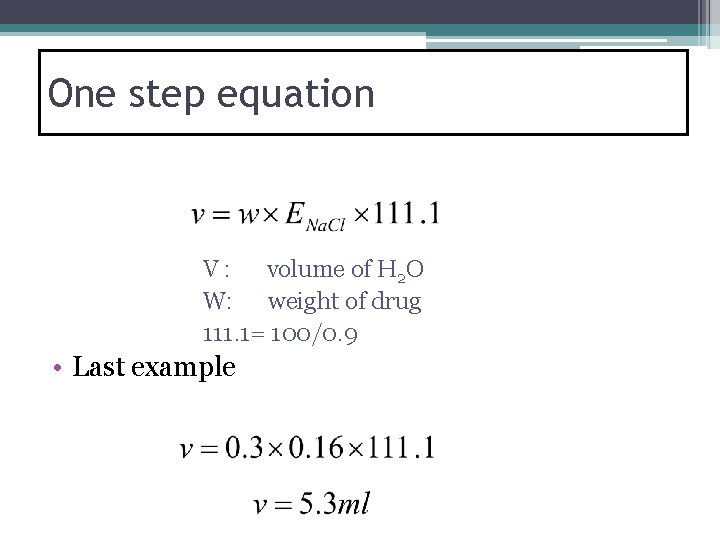
One step equation V : volume of H 2 O W: weight of drug 111. 1= 100/0. 9 • Last example

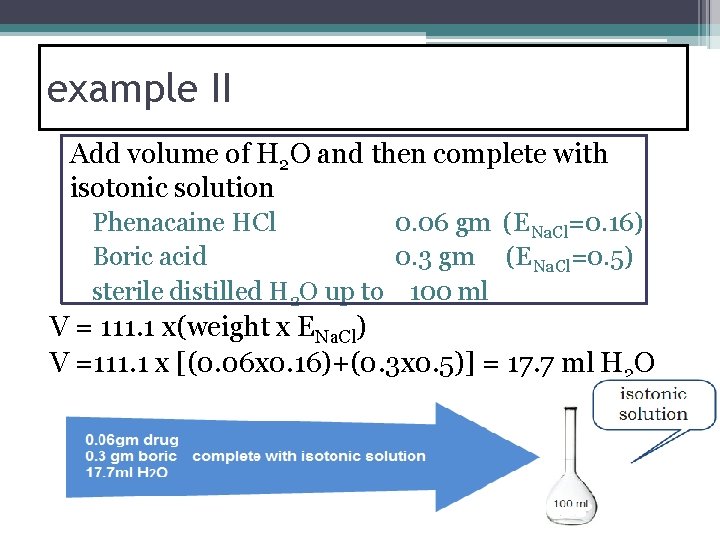
example II Add volume of H 2 O and then complete with isotonic solution Phenacaine HCl 0. 06 gm (ENa. Cl=0. 16) Boric acid 0. 3 gm (ENa. Cl=0. 5) sterile distilled H 2 O up to 100 ml V = 111. 1 x(weight x ENa. Cl) V =111. 1 x [(0. 06 x 0. 16)+(0. 3 x 0. 5)] = 17. 7 ml H 2 O
 Diffusion osmosis
Diffusion osmosis Receptor - mediated endocytosis
Receptor - mediated endocytosis Plant cells isotonic
Plant cells isotonic Types of diffusion
Types of diffusion White vincent method
White vincent method Importance of isotonic solution in pharmacy
Importance of isotonic solution in pharmacy Lattice theorem proof
Lattice theorem proof Adjustment of isotonicity
Adjustment of isotonicity Relocation diffusion vs expansion diffusion
Relocation diffusion vs expansion diffusion Facilitated diffusion
Facilitated diffusion Pressure half time formula
Pressure half time formula Pht logo
Pht logo Giá pht
Giá pht Giá pht
Giá pht S'pht
S'pht Pht mitral valve
Pht mitral valve Anatomy team 434
Anatomy team 434 Ece 434
Ece 434 Fabric testing standards
Fabric testing standards Ece 434
Ece 434 Anatomy team 434
Anatomy team 434 434 x 2
434 x 2