ISOMERS Learning Goals I will understand the difference





- Slides: 23

ISOMERS Learning Goals: I will understand the difference between structural isomers and stereoisomers (diastereomers and enantiomers) and be able to name/draw them accordingly

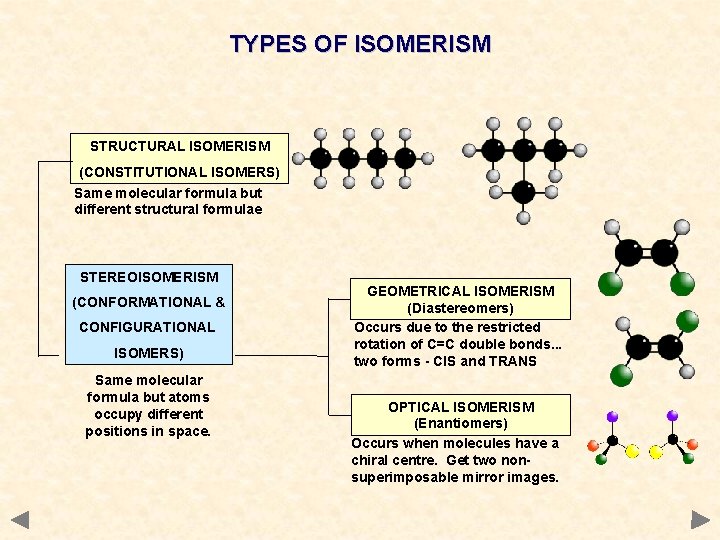
TYPES OF ISOMERISM STRUCTURAL ISOMERISM (CONSTITUTIONAL ISOMERS) Same molecular formula but different structural formulae STEREOISOMERISM (CONFORMATIONAL & CONFIGURATIONAL ISOMERS) Same molecular formula but atoms occupy different positions in space. GEOMETRICAL ISOMERISM (Diastereomers) Occurs due to the restricted rotation of C=C double bonds. . . two forms - CIS and TRANS OPTICAL ISOMERISM (Enantiomers) Occurs when molecules have a chiral centre. Get two nonsuperimposable mirror images.

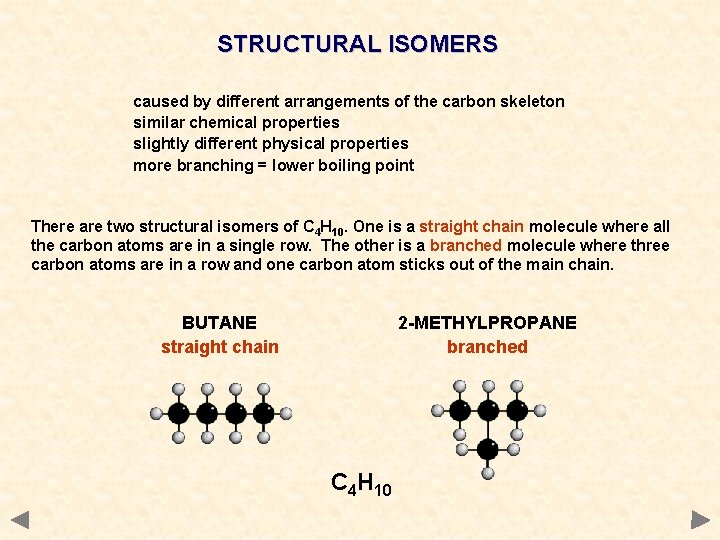
STRUCTURAL ISOMERS caused by different arrangements of the carbon skeleton similar chemical properties slightly different physical properties more branching = lower boiling point There are two structural isomers of C 4 H 10. One is a straight chain molecule where all the carbon atoms are in a single row. The other is a branched molecule where three carbon atoms are in a row and one carbon atom sticks out of the main chain. BUTANE straight chain 2 -METHYLPROPANE branched C 4 H 10

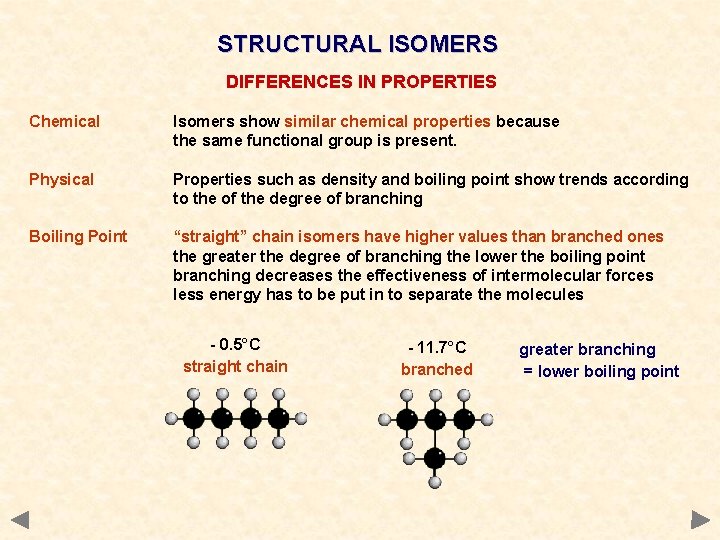
STRUCTURAL ISOMERS DIFFERENCES IN PROPERTIES Chemical Isomers show similar chemical properties because the same functional group is present. Physical Properties such as density and boiling point show trends according to the of the degree of branching Boiling Point “straight” chain isomers have higher values than branched ones the greater the degree of branching the lower the boiling point branching decreases the effectiveness of intermolecular forces less energy has to be put in to separate the molecules - 0. 5°C straight chain - 11. 7°C branched greater branching = lower boiling point

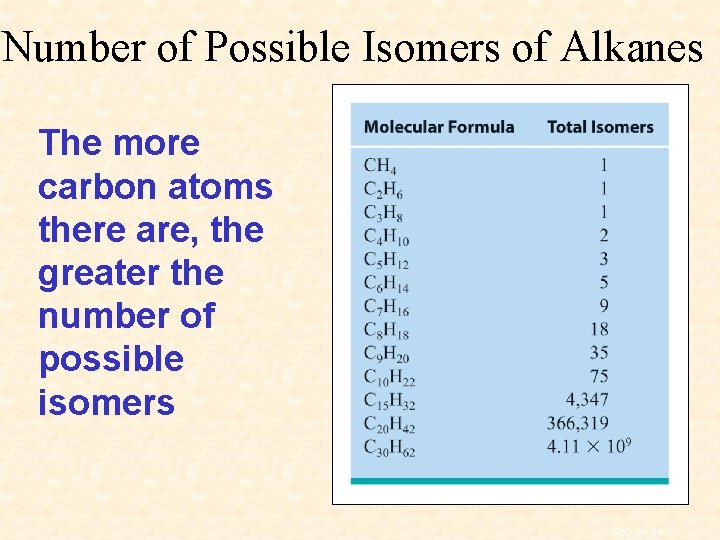
Number of Possible Isomers of Alkanes The more carbon atoms there are, the greater the number of possible isomers Section 14. 3

Drawing Structural Isomers: You Try There are 5 structural alkane isomers for C 6 H 14. Can you draw and name them all? Use molecular model kits to help. Section 14. 3

STEREOISOMERS Molecules have the SAME MOLECULAR FORMULA but the atoms are joined to each other in a DIFFERENT SPACIAL ARRANGEMENT - they occupy a different position in 3 -dimensional space. There are two types. . . • GEOMETRICAL ISOMERISM (Diastereomers) • OPTICAL ISOMERISM (Enantiomers)

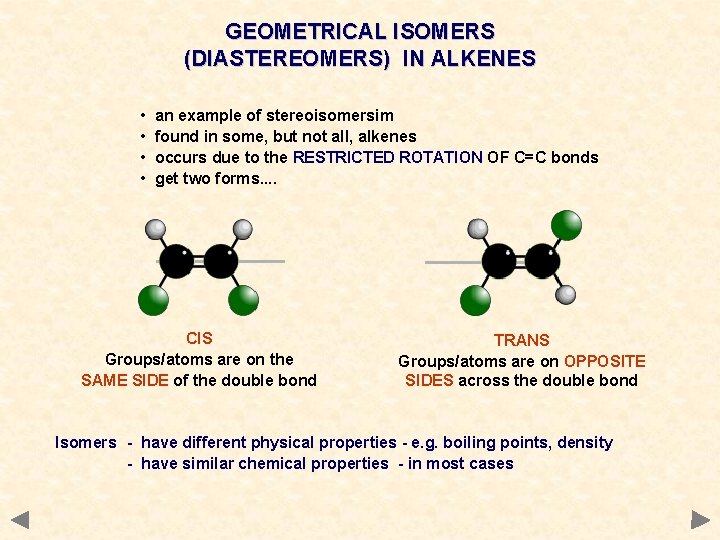
GEOMETRICAL ISOMERS (DIASTEREOMERS) IN ALKENES • • an example of stereoisomersim found in some, but not all, alkenes occurs due to the RESTRICTED ROTATION OF C=C bonds get two forms. . CIS Groups/atoms are on the SAME SIDE of the double bond TRANS Groups/atoms are on OPPOSITE SIDES across the double bond Isomers - have different physical properties - e. g. boiling points, density - have similar chemical properties - in most cases

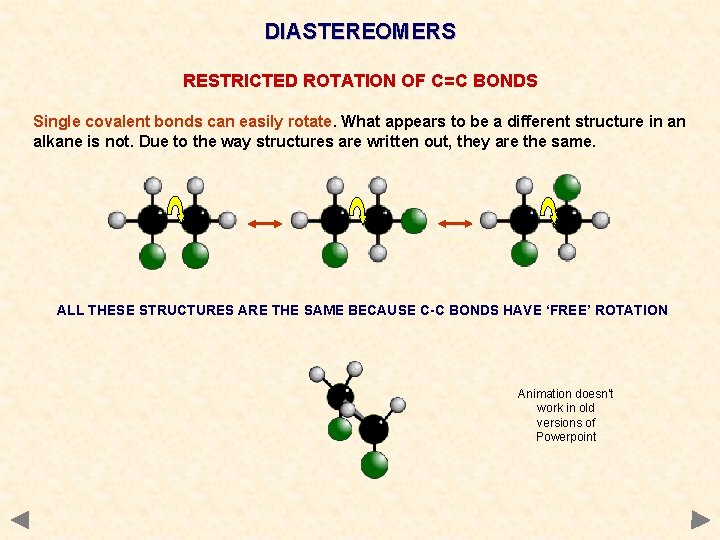
DIASTEREOMERS RESTRICTED ROTATION OF C=C BONDS Single covalent bonds can easily rotate. What appears to be a different structure in an alkane is not. Due to the way structures are written out, they are the same. ALL THESE STRUCTURES ARE THE SAME BECAUSE C-C BONDS HAVE ‘FREE’ ROTATION Animation doesn’t work in old versions of Powerpoint

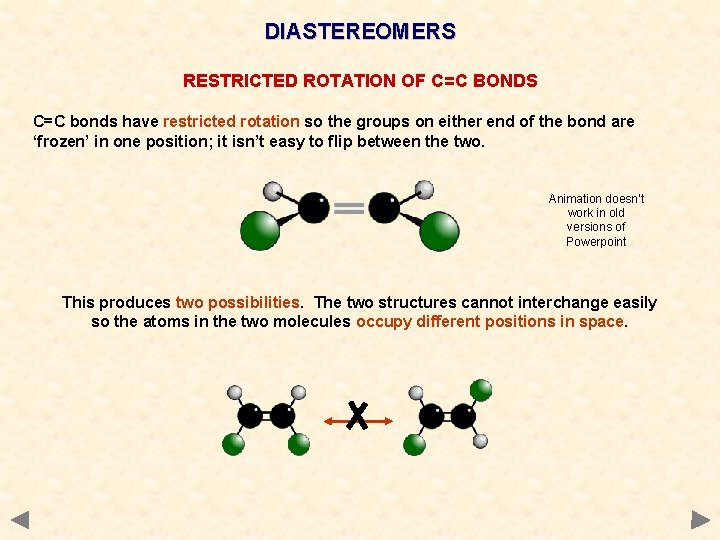
DIASTEREOMERS RESTRICTED ROTATION OF C=C BONDS C=C bonds have restricted rotation so the groups on either end of the bond are ‘frozen’ in one position; it isn’t easy to flip between the two. Animation doesn’t work in old versions of Powerpoint This produces two possibilities. The two structures cannot interchange easily so the atoms in the two molecules occupy different positions in space.

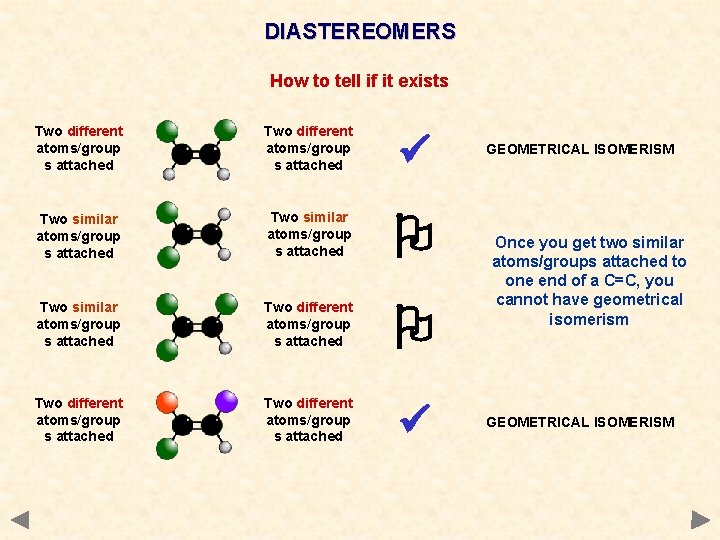
DIASTEREOMERS How to tell if it exists Two different atoms/group s attached Two similar atoms/group s attached Two different atoms/group s attached GEOMETRICAL ISOMERISM Once you get two similar atoms/groups attached to one end of a C=C, you cannot have geometrical isomerism GEOMETRICAL ISOMERISM

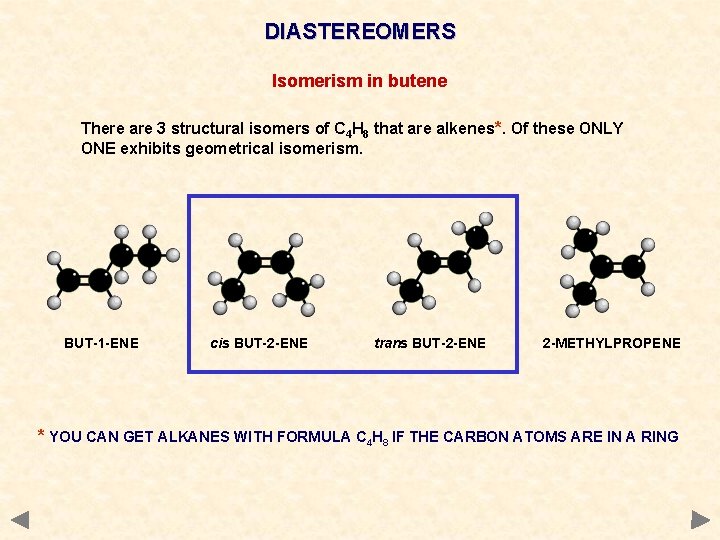
DIASTEREOMERS Isomerism in butene There are 3 structural isomers of C 4 H 8 that are alkenes*. Of these ONLY ONE exhibits geometrical isomerism. BUT-1 -ENE cis BUT-2 -ENE trans BUT-2 -ENE 2 -METHYLPROPENE * YOU CAN GET ALKANES WITH FORMULA C 4 H 8 IF THE CARBON ATOMS ARE IN A RING

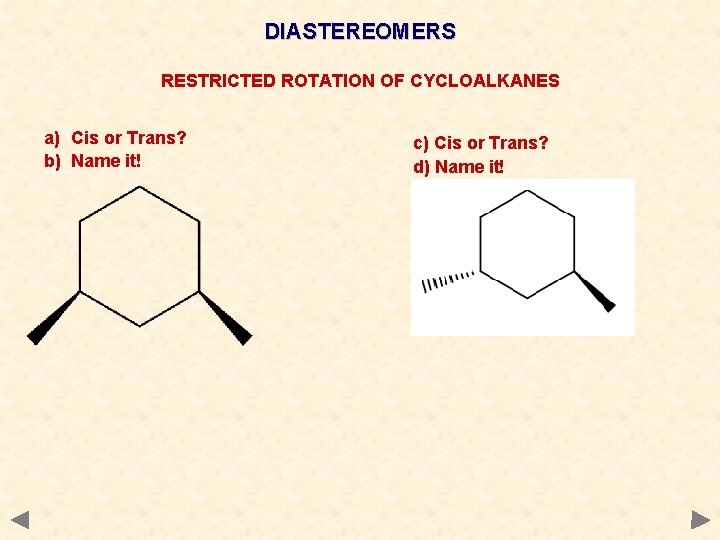
DIASTEREOMERS RESTRICTED ROTATION OF CYCLOALKANES a) Cis or Trans? b) Name it! c) Cis or Trans? d) Name it!

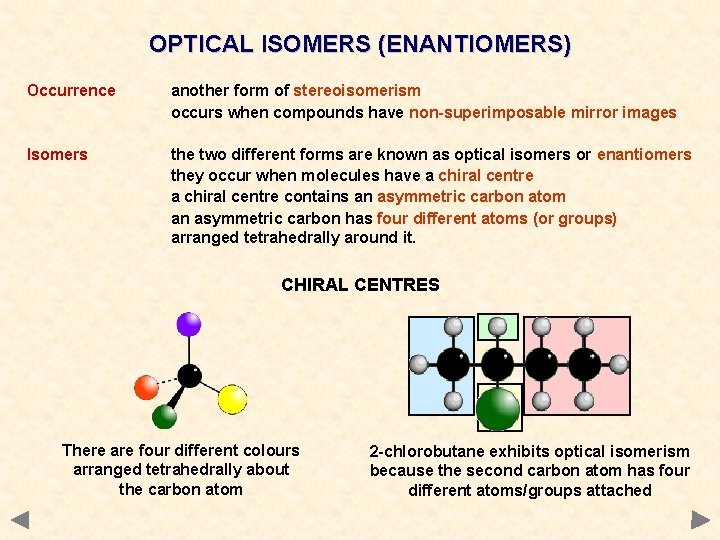
OPTICAL ISOMERS (ENANTIOMERS) Occurrence another form of stereoisomerism occurs when compounds have non-superimposable mirror images Isomers the two different forms are known as optical isomers or enantiomers they occur when molecules have a chiral centre contains an asymmetric carbon atom an asymmetric carbon has four different atoms (or groups) arranged tetrahedrally around it. CHIRAL CENTRES There are four different colours arranged tetrahedrally about the carbon atom 2 -chlorobutane exhibits optical isomerism because the second carbon atom has four different atoms/groups attached

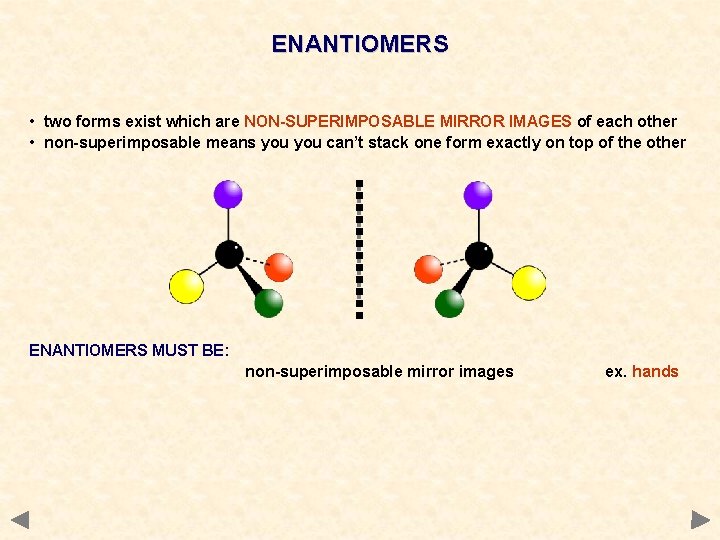
ENANTIOMERS • two forms exist which are NON-SUPERIMPOSABLE MIRROR IMAGES of each other • non-superimposable means you can’t stack one form exactly on top of the other ENANTIOMERS MUST BE: non-superimposable mirror images ex. hands

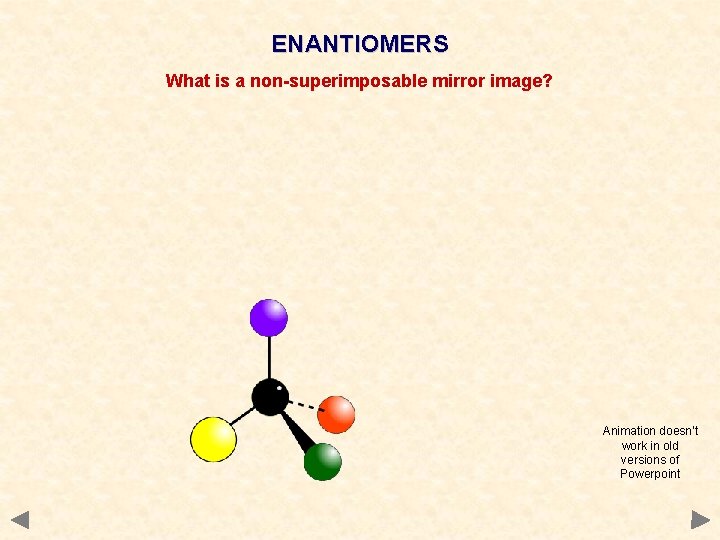
ENANTIOMERS What is a non-superimposable mirror image? Animation doesn’t work in old versions of Powerpoint

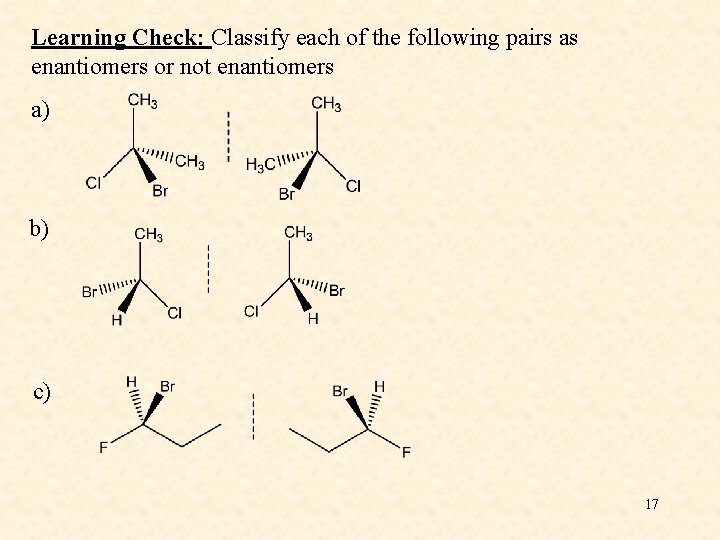
Learning Check: Classify each of the following pairs as enantiomers or not enantiomers a) b) c) 17

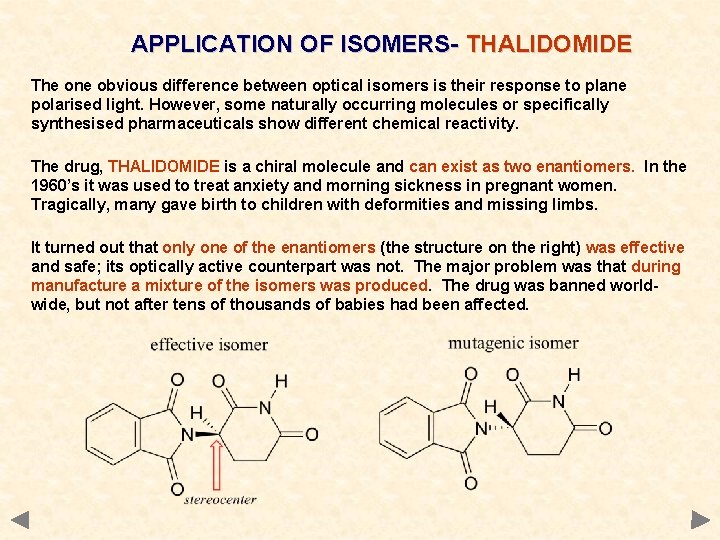
APPLICATION OF ISOMERS- THALIDOMIDE The one obvious difference between optical isomers is their response to plane polarised light. However, some naturally occurring molecules or specifically synthesised pharmaceuticals show different chemical reactivity. The drug, THALIDOMIDE is a chiral molecule and can exist as two enantiomers. In the 1960’s it was used to treat anxiety and morning sickness in pregnant women. Tragically, many gave birth to children with deformities and missing limbs. It turned out that only one of the enantiomers (the structure on the right) was effective and safe; its optically active counterpart was not. The major problem was that during manufacture a mixture of the isomers was produced. The drug was banned worldwide, but not after tens of thousands of babies had been affected.

A Quote from Lewis Carroll’s ‘Alice Through the Looking Glass’… “How would you like to live in Looking-glass House, Kitty? I wonder if they'd give you milk, there? Perhaps Looking-glass milk isn't good to drink. . . ” 19

The looking-glass world Alice entered would indeed be a very different place, with mirrorimage DNA and enzymes and amino acids, meaning that - as Alice suggested to her kitten - looking-glass milk would almost certainly not be good to drink. Its mirror-image molecules would all be the wrong way around for her enzymes to digest 20

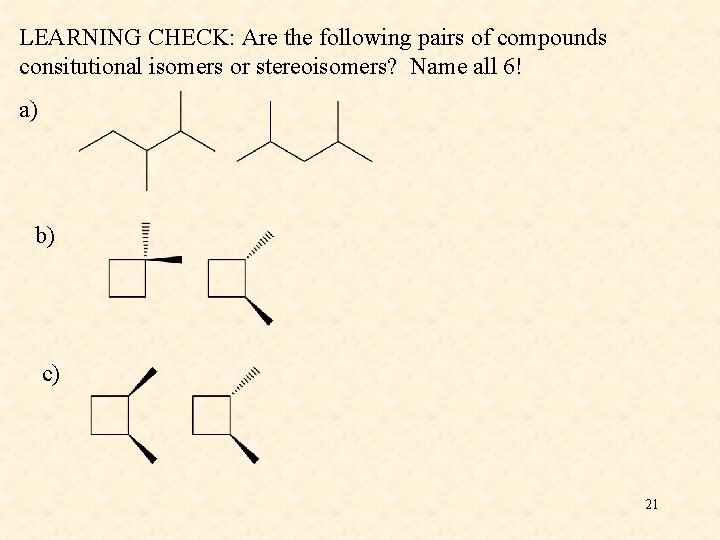
LEARNING CHECK: Are the following pairs of compounds consitutional isomers or stereoisomers? Name all 6! a) b) c) 21

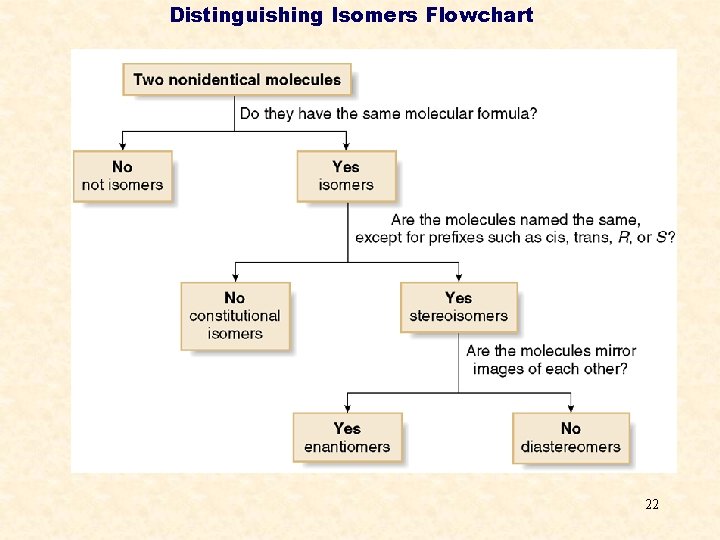
Distinguishing Isomers Flowchart 22

How Did We Do? Learning Goals: I will understand the difference between structural isomers and stereoisomers (diastereomers and enantiomers) and be able to name/draw them accordingly