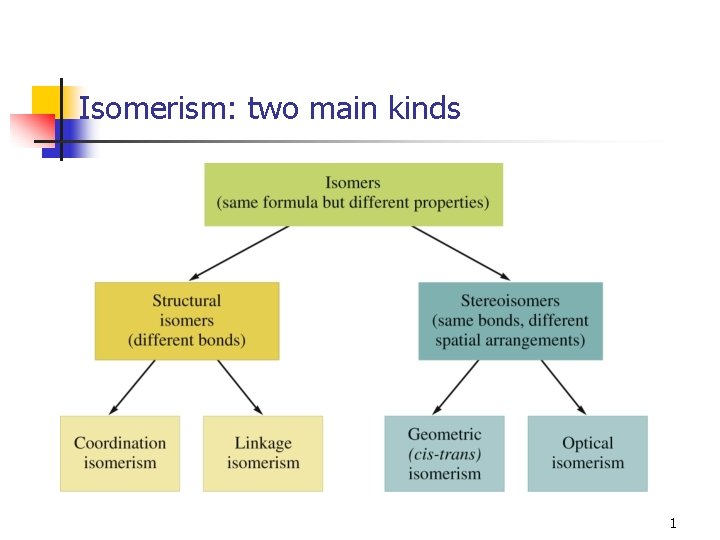
Isomerism two main kinds 1 Structural Isomers Coordination

![MO Energy Diagram for [Co(NH 3)6]3+ n n If ligands are “lone pairs”, with MO Energy Diagram for [Co(NH 3)6]3+ n n If ligands are “lone pairs”, with](https://slidetodoc.com/presentation_image_h2/a8ad43c16846476f9f67aaa895ea94b1/image-11.jpg)
- Slides: 16

Isomerism: two main kinds 1

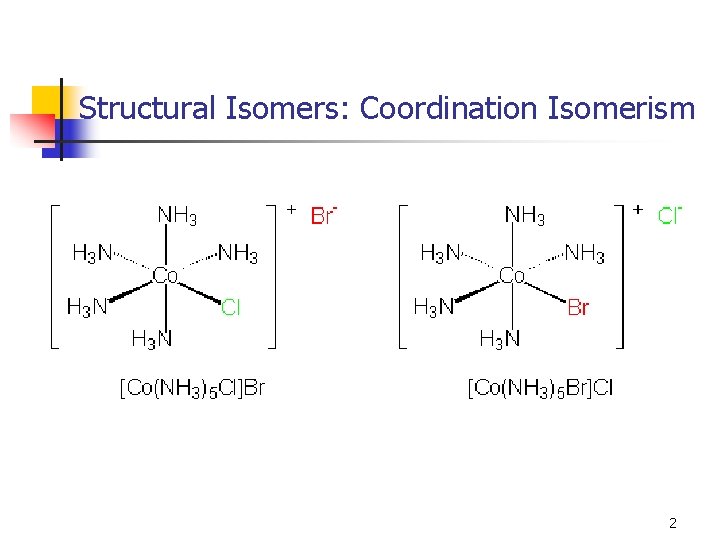
Structural Isomers: Coordination Isomerism 2

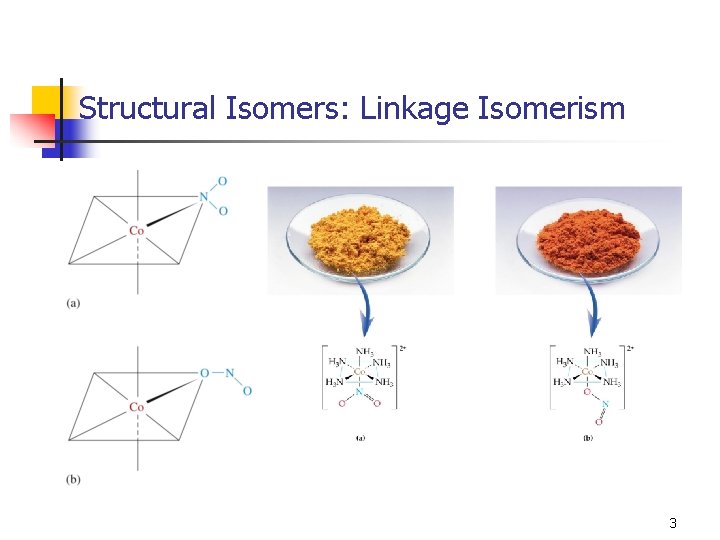
Structural Isomers: Linkage Isomerism 3

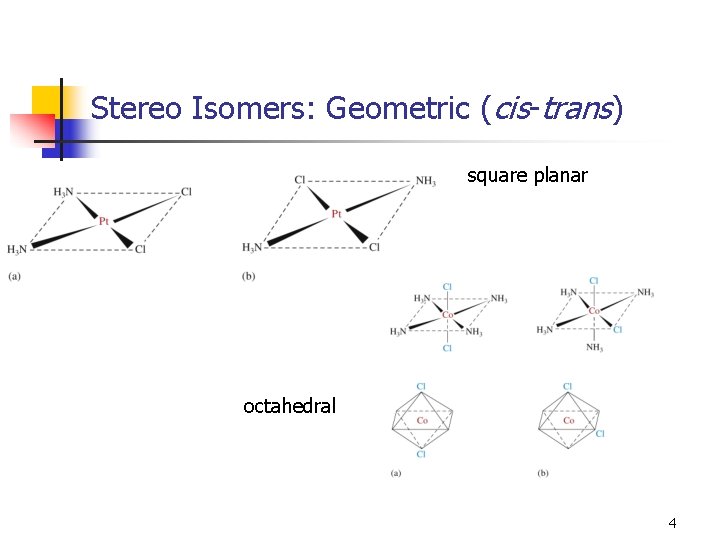
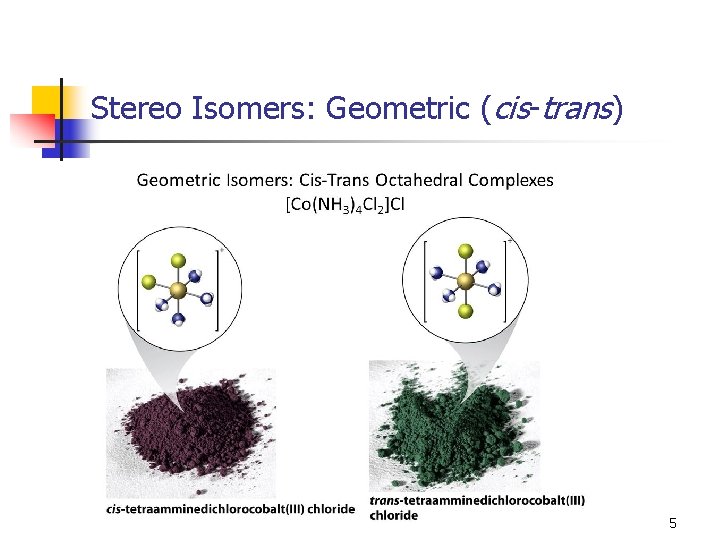
Stereo Isomers: Geometric (cis-trans) n square planar octahedral 4

Stereo Isomers: Geometric (cis-trans) 5

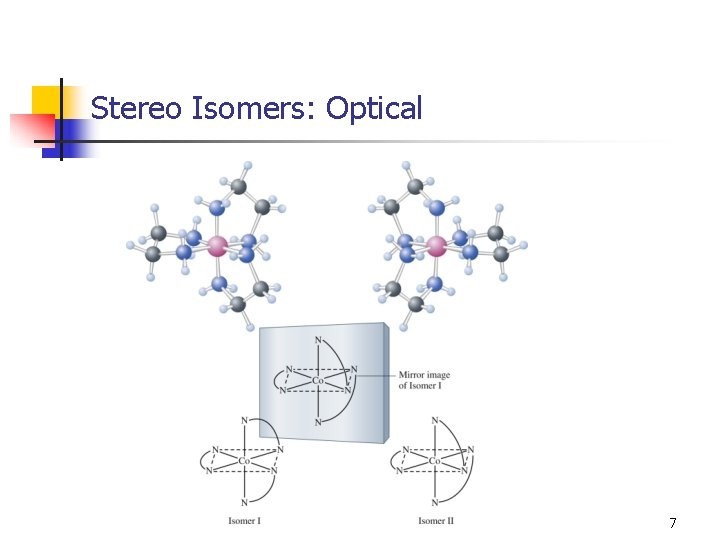
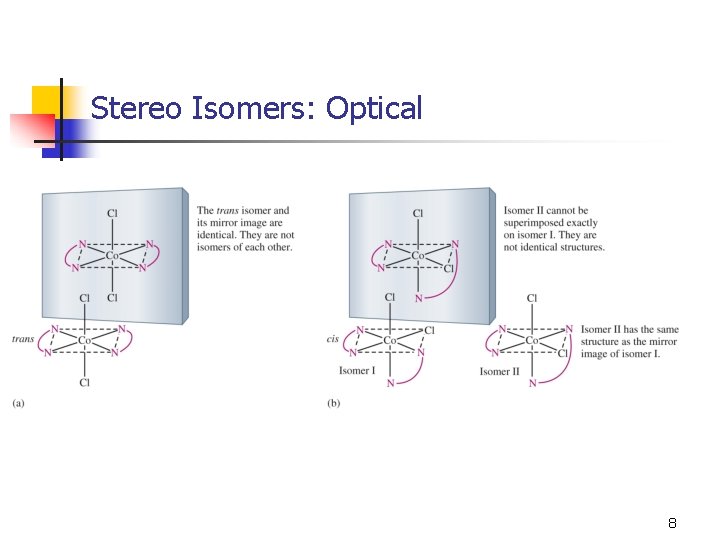
Stereo Isomers: Optical activity: opposite effects on plane-polarized light. Molecules have nonsuperimposable mirror images. 6

Stereo Isomers: Optical 7

Stereo Isomers: Optical 8

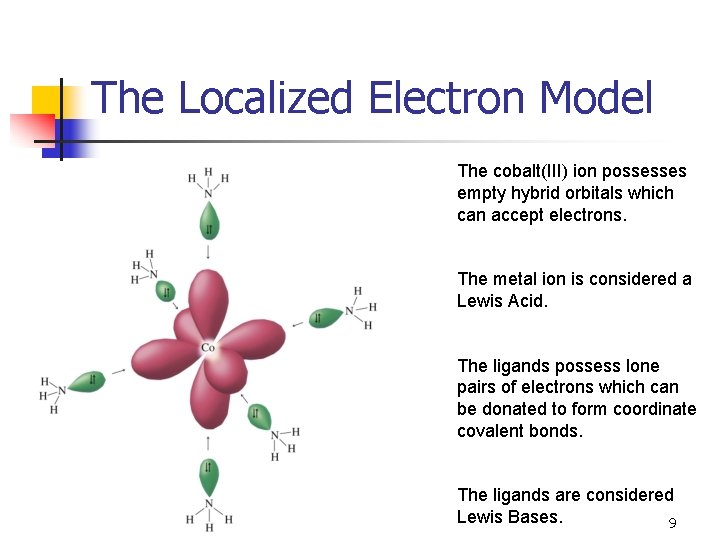
The Localized Electron Model The cobalt(III) ion possesses empty hybrid orbitals which can accept electrons. The metal ion is considered a Lewis Acid. The ligands possess lone pairs of electrons which can be donated to form coordinate covalent bonds. The ligands are considered Lewis Bases. 9

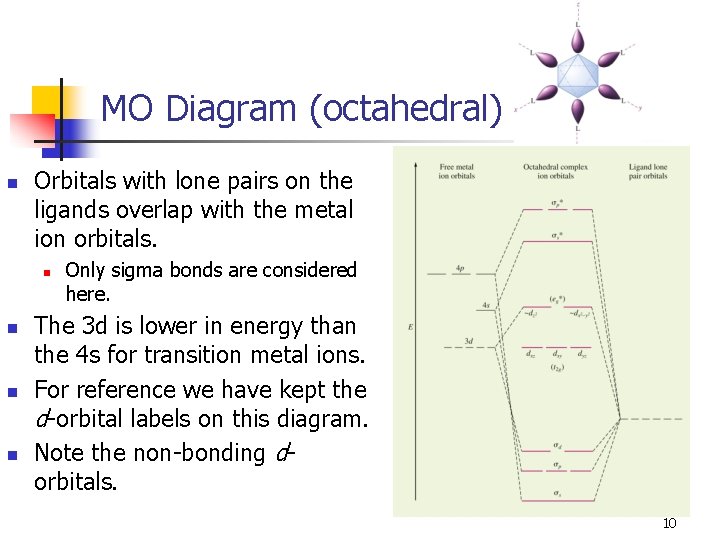
MO Diagram (octahedral) n Orbitals with lone pairs on the ligands overlap with the metal ion orbitals. n n Only sigma bonds are considered here. The 3 d is lower in energy than the 4 s for transition metal ions. For reference we have kept the d-orbital labels on this diagram. Note the non-bonding dorbitals. 10
![MO Energy Diagram for CoNH 363 n n If ligands are lone pairs with MO Energy Diagram for [Co(NH 3)6]3+ n n If ligands are “lone pairs”, with](https://slidetodoc.com/presentation_image_h2/a8ad43c16846476f9f67aaa895ea94b1/image-11.jpg)
MO Energy Diagram for [Co(NH 3)6]3+ n n If ligands are “lone pairs”, with 6 lone pairs (octahedral) we always have 12 electrons from the ligands. Thus, the number of electrons in the “dorbital” range of the MO = the number of electrons in the metal ion. 11

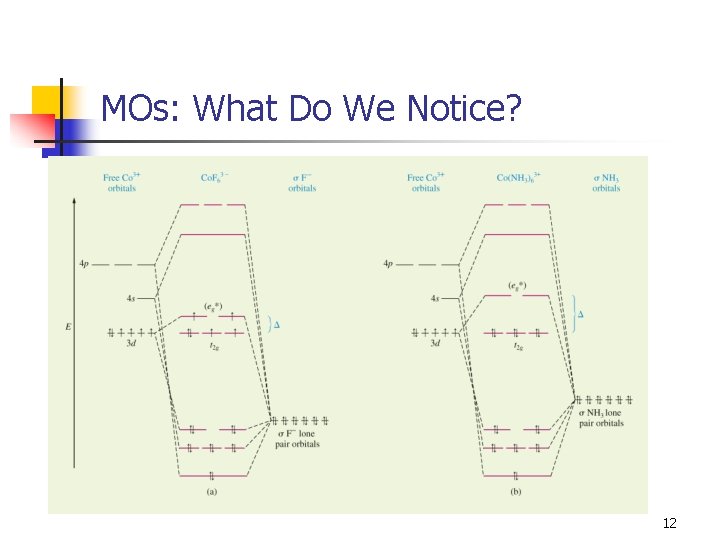
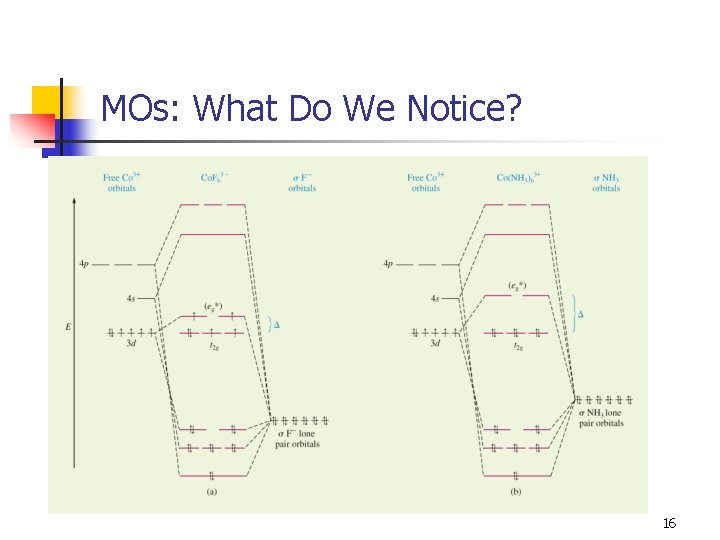
MOs: What Do We Notice? 12

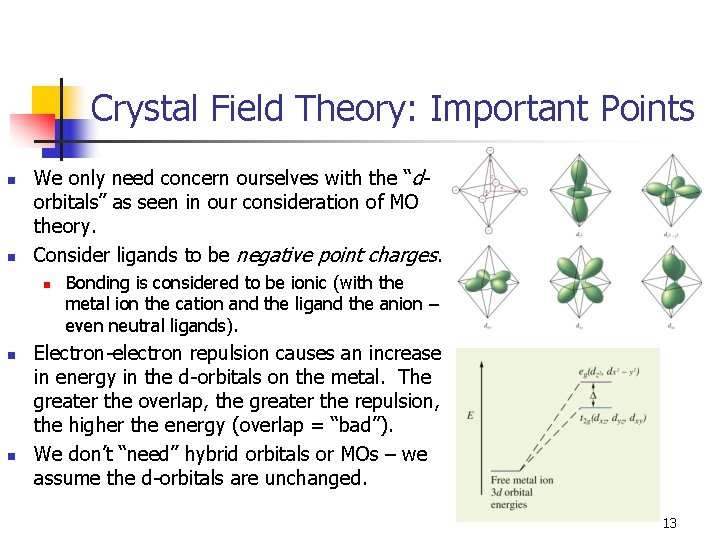
Crystal Field Theory: Important Points n n We only need concern ourselves with the “dorbitals” as seen in our consideration of MO theory. Consider ligands to be negative point charges. n n n Bonding is considered to be ionic (with the metal ion the cation and the ligand the anion – even neutral ligands). Electron-electron repulsion causes an increase in energy in the d-orbitals on the metal. The greater the overlap, the greater the repulsion, the higher the energy (overlap = “bad”). We don’t “need” hybrid orbitals or MOs – we assume the d-orbitals are unchanged. 13

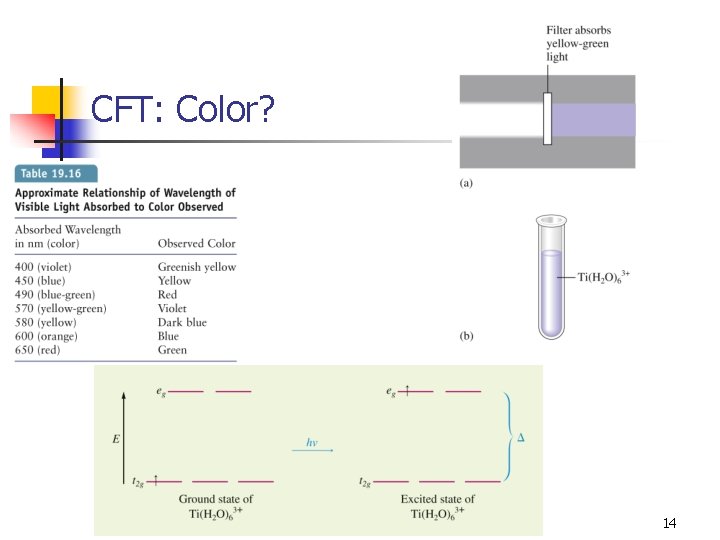
CFT: Color? 14

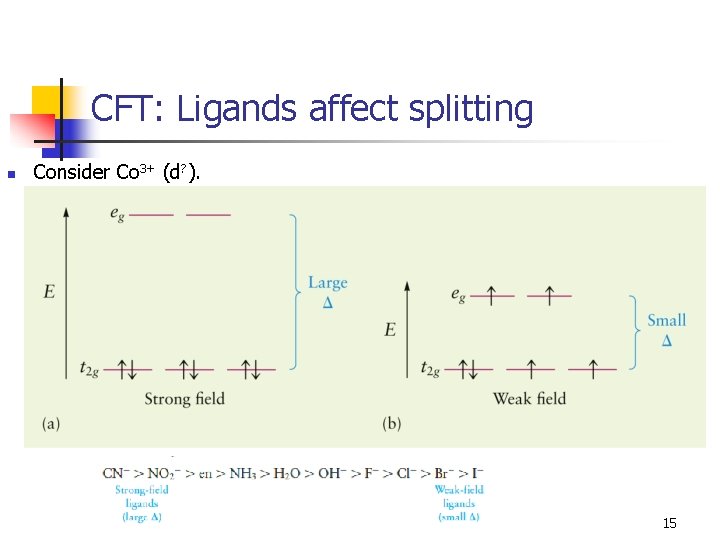
CFT: Ligands affect splitting n Consider Co 3+ (d? ). 15

MOs: What Do We Notice? 16