Isolation of Salmonella in foods Salmonella Gram negative
















- Slides: 26

Isolation of Salmonella in foods

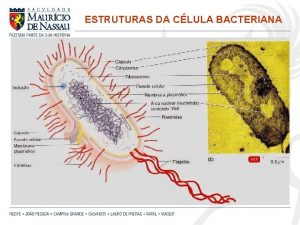
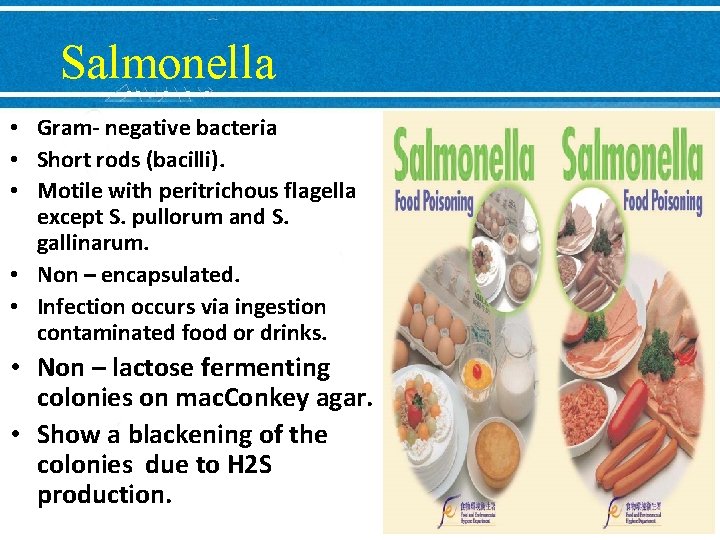
Salmonella • Gram- negative bacteria • Short rods (bacilli). • Motile with peritrichous flagella except S. pullorum and S. gallinarum. • Non – encapsulated. • Infection occurs via ingestion contaminated food or drinks. • Non – lactose fermenting colonies on mac. Conkey agar. • Show a blackening of the colonies due to H 2 S production.

Principle v The procedure consists of six distinct stages. The initial handling of the food and the non-selective enrichment stage (pre enrichment) vary according to the type of food examined. § Non-Selective Enrichment (Pre enrichment). The test sample is initially inoculated into a non-inhibitory liquid medium to favor the repair and growth of stressed or sub lethally-injured salmonellae arising from exposure to heat, freezing, desiccation, preservatives, high osmotic pressure or wide temperature fluctuations.

§ Selective Enrichment Replicate portions of each pre enrichment culture are inoculated into two enrichment media to favor the proliferation of salmonellae through a selective repression or inhibition of the growth of competing microorganism. § Selective Plating Enrichment cultures are streaked onto selective differential agars for the isolation of salmonellae. § Purification Presumptive Salmonella isolates are purified on Mac. Conkey agar plates or SS agar plates.

§ Biochemical Screening Isolates are screened using determinant biochemical reactions. § Serological Identification Polyvalent and/or somatic grouping antisera are used to support the tentative identification of isolates as members of Salmonella spp. For confirmation, cultures should be sent to a reference typing center for complete serotyping.

Collection of samples Ø Food control efforts frequently target processes and products presenting significant human health risks. The International Commission on Microbiological Specifications for Foods (ICMSF) has categorized foods according to the degree of hazard associated with product use. Ø Each food category carries an appropriately stringent sampling plan to determine the acceptability of the food product. Ø The choice of sampling plan may require some subjective judgment based on the number and kinds of factors that contribute to the degree of hazard.

Materials And Special Equipment 1. 2. 3. 4. 5. 6. 7. 8. 9. Nutrient Broth (NB). Trypticase (Tryptic, Tryptone) Soy Broth. Brilliant Green Water. Buffered Peptone Water (BPW). Skim Milk Medium. Tetrathionate Brilliant Green Broth (TBG). For the selective enrichment of Salmonella species from faeces, food and meat samples etc Selenite Cystine Broth (SC). Bismuth Sulfite Agar (BS). Brilliant Green Sulfa Agar (BGS). 10. Mac. Conkey Agar, SS agar 11. Nutrient Agar. 12. 13. 14. 15. 16. Triple Sugar Iron Agar (TSI). Lysine Iron Agar (LIA). Urea Agar (Christensen's). Commercial biochemical test kits. Polyvalent and single grouping somatic (O) and flagellar (H) antisera. 17. Physiological Saline. 18. Blender, stomacher or other homogenizing device. 19. Incubator or water bath capable of maintaining 35± 0. 5 o. C and 43± 0. 5 o. C.

Procedure Handling of Sample Units Ø Analyze samples as soon as possible. If necessary, store samples under time and temperature conditions that will prevent the growth or death of native microflora. If sample units have been abused in transit, resampling of the lot should be carried out. A. Frozen Foods: Sample units that show no signs of thawing upon receipt may be stored in the freezer at -10 o. C to -20 o. C. B. Dried and shelf stable foods may be stored at room temperature. C. Refrigerate all other foods, including those that are received in a partially thawed condition; analyze these samples as soon as possible preferably within 24 h of receipt. Ø Thaw frozen samples at room temperature within 60 min; If this is not possible, thaw the samples at refrigerator (4 to 10 o. C) temperature.

Notes: A. Large samples (e. g. whole chicken) may not readily thaw at refrigerator temperatures. For greater expediency, enclose the frozen sample in a heavy-walled paper bag and thaw overnight at room temperature. This technique maintains the product surface cold during the thawing process. B. Appropriate containers should ensure that the drippings from the product do not contaminate the laboratory environment. Ø If the sample unit received for analysis is less than the recommended analytical unit, analyze the entire amount and record the weight used. Ø Blending of samples should be limited to the minimum time required to produce a homogeneous suspension. Excessive blending could result in physical damage that would adversely affect the viability of endogenous microflora. For products that do not require blending, disperse the analytical unit into the appropriate pre enrichment broth. Ø Use aseptic techniques and sterile equipment at all stages of analysis. Containment during the handling of powdered products is critical if cross-contamination of the work environment is to be avoided.

Non-selective Enrichment (Pre-enrichment) v Compositing of Analytical Units If a sample unit consists of more than one container, aseptically mix the contents of the containers prior to withdrawal of the analytical unit. If not possible or practical, the analytical unit shall then consist of equal portions from each of the containers. v Sample Analysis The required analytical unit is dispersed into a suitable non-selective enrichment broth Nutrient broth (NB) and buffered peptone water (BPW) are equally reliable and can be used interchangeably as general purpose Pre-enrichment. If the p. H of the pre- enrichment mixture lies outside the range of 6. 0 - 7. 0, adjust with 1 N Na. OH or 1 N HCl.

NOTE: ü A positive Salmonella and a negative medium control should be set up in parallel with the test samples. Incubate the preenrichment mixture and the positive and negative controls at 35± 0. 5 o. C for 18 - 24 h. ü The negative medium control should not show any evidence of growth after incubation whereas the absence of growth in the positive control would invalidate test results.

Selective Enrichment Ø With a sterile pipette, transfer 1. 0 m. L of the pre- enrichment culture into each of 9 m. L of selenite cystine (SC) and tetrathionate brilliant green (TBG) broths. Ø Incubate SC and TBG broths for 24± 2 h at 35± 0. 5 o. C and 43± 0. 5 o. C, respectively.

Selective Plating Ø Streak replicate loopsful of each selective enrichment culture onto BS and BGS agar to obtain well isolated colonies. The enrichment cultures may be streaked onto additional plating media for the isolation of Salmonella. Incubate plates at 35± 0. 5 o. C for 24± 2 h. If colonies suggestive of Salmonella have not developed on BS plates, incubate for an additional 24± 2 h. Ø Examine incubated plates for colonies suggestive of Salmonella. Typical Salmonella usually occur as pink to fuchsia colonies surrounded by red medium on BGS agar, and as black colonies on BS agar with or without a metallic sheen, and showing a gradual H 2 S- dependent blackening of the surrounding medium with increasing incubation time.

ü NOTE: Ø Lactose-and/or sucrose-fermenting Salmonella strains develop a coliform-like (greenish) appearance on BGS agar. A heavy growth of non-salmonellae may also mask the presence of Salmonella on this medium.

Purification Ø Streak suspect colonies onto Mac. Conkey agar for purification. Incubate plates at 35± 0. 5 o. C for 24± 2 h. Ø Typical Salmonella colonies are lactose-negative and will appear as colorless colonies on this medium. Ø However, lactose-positive biotypes will occur as pink colonies.

Biochemical Screening Ø With a sterile needle, inoculate suspect colonies into the biochemical media or in commercial diagnostic kits that would yield equivalent results. Ø Incubate the biochemical media for 18 -24 h at 35± 0. 5 o. C. ØCommercially available diagnostic kits may be used to obtain detailed biochemical profiles of bacterial isolates. If none of the isolates from a particular analytical unit are suggestive of Salmonella, the analytical unit is considered to be free of salmonellae. If the presence of Salmonella is suspected, proceed with serological testing. If serological testing is not to be performed within 72 h, inoculate suspect isolates into nutrient agar slants and incubate at 35± 0. 5 o. C for 24± 2 h. Store the agar slants at refrigerator (4 to 10 o. C) temperature. Nutrient agar slants that have been stored for more than 72 h should not be used for serological testing. Prepare fresh agar slants for this purpose.

Serological Identification v Testing with somatic polyvalent antiserum Ø Mark the following sections on an agglutination plate: C+ (positive control), C- (negative control) and T (test culture). Ø Add one drop of physiological saline to each of the areas marked T and C+, and two drops to the area marked C-. Ø Remove sufficient culture material from a triple sugar iron, lysine iron or nutrient agar slant to prepare a heavy suspension in the test area (T) and in the negative control (C-) area. The inoculum should be withdrawn from the slope portion of agar slants.

Ø For the positive control, prepare a heavy suspension of a known Salmonella culture in the area marked C+. Ø Prepare somatic polyvalent antisera as directed by the manufacturer; add one drop to each of the areas marked T and C+. Ø Mix the culture-saline-antiserum suspensions in T and C+ and the saline-culture mixture in C- with a sterile needle or loop. Tilt the slide preparation back and forth for 1 min. Ø Hold the slide against a dark background and observe for agglutination. Salmonella cultures usually agglutinate within 1 min. Ø False positive reactions from microorganisms that are closely related to Salmonella may occur. Such misleading reactions can be resolved through further testing with somatic grouping and flagellar antisera. Ø The serological test for a given culture is invalidated if the negative control shows agglutination (auto-agglutination).

v Testing with Somatic Grouping Antisera Ø It is advantageous to test presumptive Salmonella cultures with somatic grouping antisera whenever possible. Many foodborne Salmonella belong to somatic groups B, C, D, or E. Ø it is important to recognize that unless a complete set of grouping antisera is available, Salmonella belonging to uncommon serogroups may be missed. ü NOTE: It should be stressed that any non-agglutinating culture possessing the biochemical reactions suggestive of Salmonella should be sent to a reference typing center for identification. Ø Mark the following sections on an agglutination plate: C-(negative control) and T (test culture). Ø If a Salmonella control culture is available for each somatic group tested, prepare C+ (positive control).

Ø Add one drop of physiological saline to each of the areas marked T and C+, and two drops to the area marked C-. Ø Remove sufficient culture material from a triple sugar iron, lysine iron or nutrient agar slant to prepare a heavy suspension in the test area and in the negative control area. The inoculum should be withdrawn from the slope portion of the agar slants. Ø Prepare somatic group antiserum as directed by the manufacturer; add one drop to each of the areas marked T and C+. Ø Mix the culture-saline-antiserum suspensions in T and C+ and the salineculture mixture in C- with a sterile needle or loop. Tilt the slide preparation back and forth for 1 min. Ø Hold the slide against a dark background and observe for agglutination. Salmonella cultures usually agglutinate within 1 min. Ø If the culture-saline-antiserum mixture does not agglutinate, repeat the procedure with another somatic group antiserum.

Ø If the serological test is positive, the culture should be sent to a Salmonella typing center for complete serotyping. Ø The serological test for a given culture is invalidated if the negative control shows agglutination (auto-agglutination). Ø A biochemically suspect Salmonella isolate that fails to yield any positive serological reaction should be sent to a reference typing center for identification. v Testing with Flagellar (H) Antisera Ø In instances where the services of a reference typing center are not available, Salmonella isolates agglutinable with somatic antisera should be further identified by testing with polyvalent H antiserum.

Bismuth Sulfite Agar is used for the isolation of S. typhi and other Salmonella spp. from food, feces, urine, sewage, and other infectious materials. Bismuth Sulfite Indicator and Brilliant Green are complementary, inhibiting Gram-positive bacteria and coliforms, allowing Salmonella spp. to grow. Ferrous Sulfate is used for H 2 S production. When H 2 S is present, the iron in the formula is precipitated, and positive cultures produce the characteristic brown to black color with metallic sheen. Brilliant Green Agar w/ Sulfapyridine is used for the selective enrichment of Salmonella spp. Lactose and Sucrose are the carbohydrates. In the presence of Phenol Red, a p. H indicator, non-lactose and/or nonsucrose-fermenting Salmonella spp. will produce pink to red colonies. Sodium Sulfapyridine and Brilliant Green are the selective agents, inhibiting Gram-positive organisms and many Gram-negative bacteria, except Salmonella.


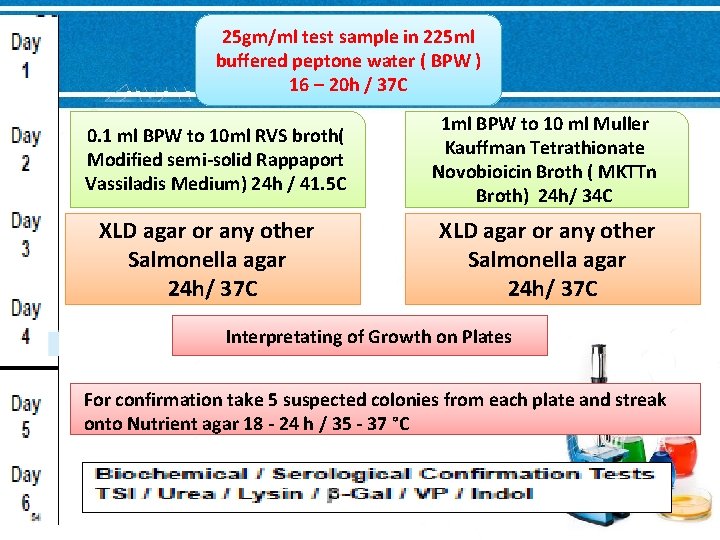
25 gm/ml test sample in 225 ml buffered peptone water ( BPW ) 16 – 20 h / 37 C 0. 1 ml BPW to 10 ml RVS broth( Modified semi-solid Rappaport Vassiladis Medium) 24 h / 41. 5 C XLD agar or any other Salmonella agar 24 h/ 37 C 1 ml BPW to 10 ml Muller Kauffman Tetrathionate Novobioicin Broth ( MKTTn Broth) 24 h/ 34 C XLD agar or any other Salmonella agar 24 h/ 37 C Interpretating of Growth on Plates For confirmation take 5 suspected colonies from each plate and streak onto Nutrient agar 18 - 24 h / 35 - 37 °C


END OF LECTURE
 Fase formula
Fase formula Perbedaan bakteri gram positif dan negatif
Perbedaan bakteri gram positif dan negatif Parede celular
Parede celular Grams to gram
Grams to gram Ons ke gram
Ons ke gram Diagram 3 fase
Diagram 3 fase Anfitríquio
Anfitríquio Flagelo
Flagelo Eubatteri
Eubatteri Tekanan uap larutan
Tekanan uap larutan Did isolation have a positive or negative effect on japan?
Did isolation have a positive or negative effect on japan? Gram
Gram Spirochete infection
Spirochete infection Gram positive rods chinese letters
Gram positive rods chinese letters Micrococcus luteus on msa
Micrococcus luteus on msa Sar of penicillin
Sar of penicillin Monotrichous
Monotrichous Streptococcus gram negative
Streptococcus gram negative Gram negative bacteria
Gram negative bacteria Enteric gram negative rods
Enteric gram negative rods Diplococcus gram negatif
Diplococcus gram negatif Gram-negative diplococci
Gram-negative diplococci Gram negative spiral shaped bacteria
Gram negative spiral shaped bacteria Gram negative diplococci
Gram negative diplococci Enteric gram negative bacilli
Enteric gram negative bacilli Gram negative
Gram negative Haemophilus influenzae septic arthritis
Haemophilus influenzae septic arthritis