Isolate storage packaging and transportation 1 Content outline




























- Slides: 33

Isolate storage, packaging and transportation 1

Content outline • • • Principle Material required and Suitable storage containers Preservation methods Sample & Evaluation of specimen quality Environmental and safety controls Procedure for Harvesting Pure Isolate Labelling and Understand proper Packing Transport of specimens Record Keeping 2

Learning objectives At the end of this module you will be able to: Ø Understand perform harvesting of Pure Culture Isolates from Positive Liquid or Solid TB Culture. Ø Understand suitable containers for the collection and storage of Culture Isolates. Ø Understand proper labelling of Culture Isolates to be sent to the National TB laboratory. Ø To describe the procedures required for the preservation of culture by (a) 10% skim milk solution (b) 25% glycerol containing Middle brook 7 H 9 medium. Ø Understand proper Packing , Labeling and transportation of Category A Biological Substance. Ø Keeping records laboratory register. 3

Principle • Mycobacterial strains must be stored in conditions that preserve their viability and protect specific strain characteristics. • Mycobacterial strains are to be stored in adequate conditions to preserve their viability and intact genetic background. • The Over all goal of this training is to describe conditions for optimal maintenance and storage of mycobacterial strains. • Unless Isolates are collected with the utmost care and promptly transported to the laboratory under the proper conditions, the advantages of culture will not be fully realized. 4

Factor that Affect Viability of MTB isolates • The microbial viability of TB strains depends on 2 factors A. the storage temperature and B. the suspending medium. • The storage temperature is more important for the prolonged survival of mycobacteria stored at sub-zero temperatures. The viability of tubercle bacilli declines much more rapidly at – 20 C than at – 70 C: only 1% are still viable at – 20 C after 2 years compared with 100% at – 70 C. • It is thus crucial to prepare the heaviest bacterial load possible (greater than a 1. 0 Mc. Farland standard) in order to compensate for the loss of viability. Note: Maintenance of mycobacterial cultures by re-inoculations of media during prolonged time may cause accumulation of mutations that can lead to unpredictable results 5

Material required • Sterile Cryotube with screw-caps • Easily-labelled walls • Leak proof • 1. 8 -2 ml capacity • Translucent or clear material • Disposable loop • • Sterile plastic pipette Rack Wash bottle Absorbent paper towel N-95 Mask Disinfectant of tuberclosidal Biological safety cabinet 6

Preservation methods for Culture Isolates • Sterile 10% skim milk medium. Prepared by mixing 100 ml skim milk powder with 1 L distilled water and autoclaved at 121*C for 15 min. Note : can be used to preserve TB isolates for 2 year at -20*C /For 10 year at -80*C. • Middle brook 7 H 9 broth enriched with OADC supplement. Can be used directly from the tube by dispensing required amount of 1. 5 to 2 ml for each vial. 7

What OADC supplement do ? • Oleic Acid – Important in mycobacterial metabolism • Albumin (bovine) – Binds free fatty acids which may be toxic to mycobacteria • Dextrose – Energy source • Catalase – Destroys toxic peroxides • Polyoxyethylene stearate (POES) – Enhances growth of M. tuberculosis and assists in providing a uniform inoculum 8

Sample • MTB Positive cultures • from Liquid and Solid Culture • with the right culture Age < 56 days • contaminated by normal flora Not Suitable. • NTM not suitable 9

TB or not TB ? M. tb complex Or • Non-Tuberculous Mycobacteria (NTM) • 1. Photo chromogens • Colonies are non-pigmented in the dark but turning lemon yellow after exposure to light • 2. Scotochromogens • Colonies are yellow to orange when grown in the light or the dark • 3. Non photochromogens • Usually non-pigmented and are unaffected by light • 4. Rapid Growers • Organisms are fully mature in 2 -5 days 1. 2. 3 4.

Appearance and colony morphology of M. bovis on LJ slants • Typical colonies of M. bovis on LJ slant have slightly different growth compared to TB – – – Small, round, Transparent, Wrinkled surface Irregular thin margins Smooth with a slightly granular surface, White • Addition of 0. 5% pyruvate (instead of glycerol) to the LJ medium facilitates the growth of M. bovis

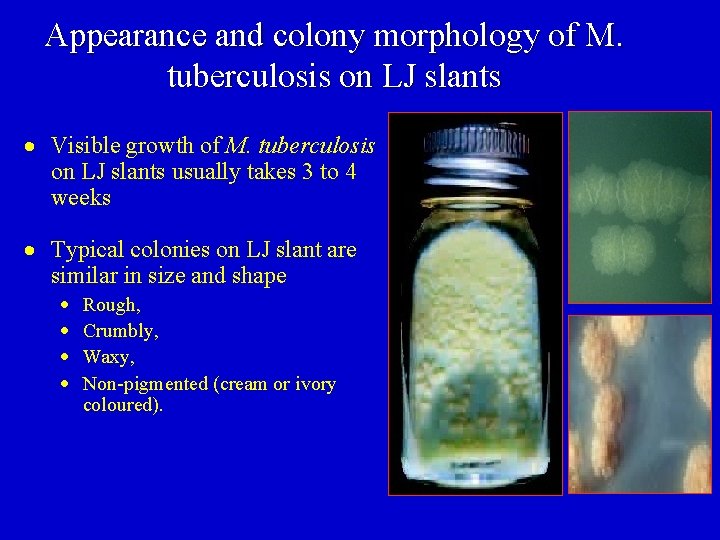
Appearance and colony morphology of M. tuberculosis on LJ slants Visible growth of M. tuberculosis on LJ slants usually takes 3 to 4 weeks Typical colonies on LJ slant are similar in size and shape Rough, Crumbly, Waxy, Non-pigmented (cream or ivory coloured).

Preliminary identification of the M. tuberculosis complex • Examine growth positive solid media cultures for the characteristic colony morphology of the M. tuberculosis complex as previously described • In case of growth detection prepare ZN smears to confirm the presence of AFB • Use of ZN (not Fluorescent stain) to see if contaminating organisms are present. • Do ID[SD or Capilla Neo Ag Test] for ZN positive Isolates.

For Liquid Culture • Instrument Positive Cultures • Remove the tube from the instrument • Scan the barcode • Visually check tube for flaky, clumped growth • Prepare smear in BSC using albumin. • Perform AFB Smear using ZN stain • Confirm presence of corded AFB • Do ID[SD or Capilla Neo Ag Test] for ZN positive Isolates. 14

Smear to confirm presence of AFB • Remove a small amount of culture from a growth positive LJ slant and gently rub it into 1 drop of sterile saline Albumin as fixative to the slide to ensure that cells are not washed off during staining. • OR transfer a 0. 1 m. L aliquot from the bottom of unmixed liquid media tube to a Albumin fixed slide. • Members of the M. tuberculosis complex microscopically show serpentine cords of varying length or linear clumping. • Corded AFB smear-positive colonies or aliquots from a liquid culture are considered presumptive positive. • Cultures should be checked for purity and identification of M. tuberculosis should be confirmed promptly using a validated identification procedure.

Environmental and safety controls • Use appropriate personal protective equipment while performing this procedure. • Use appropriate disinfectant with the right concentration and age. • perform the procedure under a Biological Safety Cabinet Class II. • Dispose after autoclaving all contaminated disposables. 16

Procedure to harvest MTB Isolates Short term Storage • Do necessary identification work up and confirm the purity. • Ensure growth is consistent with MTB prior to storing. • Store isolates at room temperature in the dark (preferred) or at 2– 8*C in the refrigerator until you do harvesting. • Store all isolates grown on the LJ or MGIT culture in numerical order based on Laboratory study ID or code. During Harvesting • For Liquid Culture : Vortex the tube for at least for one minutes. Make sure the suspension is well dispensed and very turbid (greater than a 1. 0 Mc. Farland standard) Let the suspension stand undisturbed for 20 minutes. • For Solid Culture: Use colonies showing good, confluent and pure growth of M. tuberculosis on LJ slant within 10 -15 days of first appearance. Older cultures will not provide reliable long term viability. 17

Cont… • Label cryotube with Laboratory code or Strain type. Use permanent label markers or computer-generated stickers that do not come off after thawing. • Check the label on the LJ tube and the cryo tube to ensure they match. • Using a sterile loop, Carefully scrape several colonies off the slant without taking any culture medium (this will give false turbidity) • Transfer growth into a screw cap tube containing 1. 5 -2 ml sterile Skim milk medium or 7 H 9 broth. • For Liquid Isolates: Dispense 1. 0 - 1. 2 ml of the suspension into cryotube using a sterile transfer pipette, without taking any sediment, Let stand this undisturbed for 15 minutes. • Place cryotube in cryobox and freeze box in a rack in a -20*C freezer. • Record the location and strain identifiers in the -80 C Freezer Storage Logbook. • Record data for each specimen on the Storage Log for M. tuberculosis Isolates. 18

Recording of growth positive results • Immediately record culture results (positivity, negativity or contamination) in the laboratory register. • Growth on solid medium should be recorded in a semi-quantitative format: If no growth is seen report “Negative” If 1 -19 colonies are seen report “Positive” and number of colonies If 20 -100 colonies are present report “Positive (1+)” If 100 -200 colonies are counted report “Positive (2+)” If 200 -500 colonies are seen report “Positive (3+)” If more than 500 colonies are seen report “Positive (4+)” If cultures are contaminated report “Contaminated” • Liquid medium Print out data from the MGIT must be reviewed and archived. • Always correlate the accession number on the tube with the number and other patient information in the laboratory register. • Record the date of culture result reported • Record the name or initials of the person reporting the result.

Transport of TB infectious substances 20

Transport: national regulations • provided that all necessary testing has been completed and all frozen stock aliquots have been appropriately stored you can arrange transportation. • To be customized according to the country’s specific rules for transport of infectious substances affecting humans. 21

? Classification of infectious substances for the purposes of transport. As Category A and B. To what category TB belong to ? • If it is Sputum and • Culture isolates 22

Transport of TB infectious substances Classification of infectious substances for the purposes of transport, as per TB: • Category A: Cultures of M. tuberculosis. For surface transport, when cultures are intended for diagnostic or clinical purposes, they may be classified as category B. For surface transport there is no maximum quantity per package. • Category B: Other infectious substances. • Guidance on regulations for the transport of infectious substances 2007– 2008. Geneva, World Health Organization, 2007 (WHO/CDS/EPR/2007. 2) available at http: //www. who. int/csr/resources/publications/biosafety/WHO_CDS_EPR_2007_2/en/index. html). 23

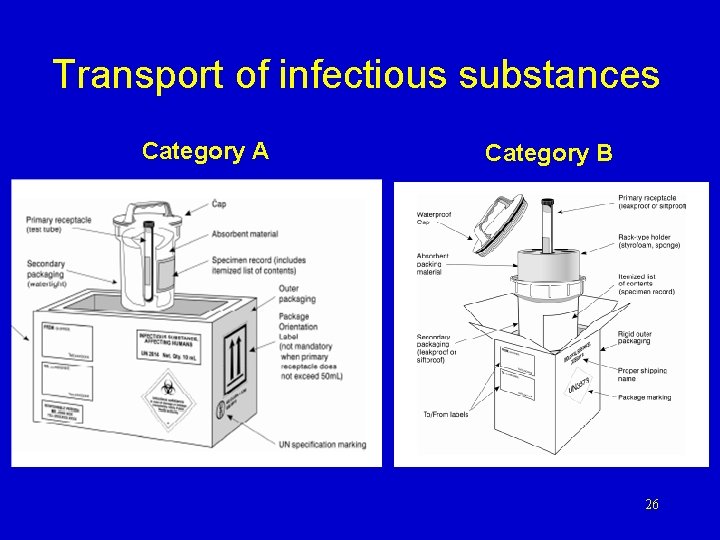
Triple Packaging (UN 2007) Category A Category B • leakproof primary • leakproof secondary • 95 k. Pa pressure test • rigid outer 6 • minimum 100 mm • absorbent material • 9 meter drop test • 7 kg penetration test • UN marks and labels • full documentation • Currier training • leakproof primary • leakproof secondary • 95 k. Pa pressure test • rigid outer • minimum 100 mm • absorbent material • 1. 2 meter drop test • 3373 label Exempt human or animal specimens • leakproof primary • leakproof secondary • adequate outer • minimum 100 mm • absorbent material UN 3373 24

Transport of infectious substances Basic triple packaging system • (i) a leak-proof primary receptacle(s); • (ii) a leak-proof secondary packaging containing sufficient additional absorbent material shall be used to absorb all fluid in case of breakage. For cold transportation conditions, ice or dry ice shall be placed outside the secondary receptacle. Wet ice shall be placed in a leak proof container. • (iii) an outer packaging of adequate strength for its capacity, mass and intended use with the right label. 25

Transport of infectious substances Category A Category B 26

Surface transport : Example of a secondary packaging 1* 2* 2* 3* 27

Air transport • For national flights (within one country) national civil aviation authorities apply national legislation. • For international flights: International Air Transport Association (IATA) www. iata. org 28

Receipt of incoming specimens 1. Inspect the delivery box for signs of leakage. 2. Disinfect the outside of the delivery box. 3. Open carefully and check for cracked or broken specimen containers. 4. Disinfect the inside of the box. 29

Identification of specimens before processing – critical issues • Request forms must be separate from specimen containers. • Each container should be clearly labelled on the side – not on the cap. • Accompanying list should include the requested data for each patient. • The number of specimen containers in the box must correspond to the number of names on the accompanying list. • The identification number on each specimen container must correspond to the identification number on the accompanying list. • The date of dispatch and the name of the Refereeing Facility or Laboratory must be included on the accompanying list. 30

Hand on practices on the following Document. . Laboratory register : culture &DST Laboratory request forms for culture and DST Sample transport log Sample recive log 31

Module review: take home messages Ø Good quality Isolate are the cornerstone for high quality tuberculosis diagnosis. Ø The quality of incoming specimens should be evaluated and recorded. Ø Proper collection procedures and containers, adequate volumes and appropriate specimen transport conditions can all affect TB culture and DST results. Ø Packaging of infected specimens that are to be sent by air or surface mail must be carried out according to national biosafety guidelines or international rules. Ø Correct labelling of specimens is critical for their identification. 32

Thank you 33