Isobaric Pressure Isobaric Expansion W Isobaric Compression W
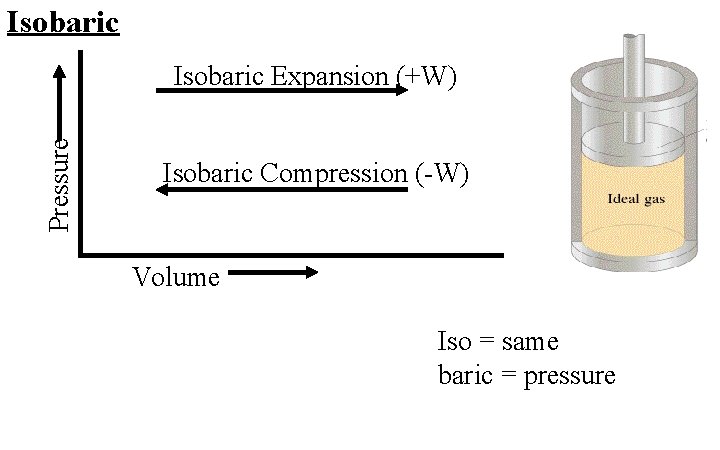
Isobaric Pressure Isobaric Expansion (+W) Isobaric Compression (-W) Volume Iso = same baric = pressure
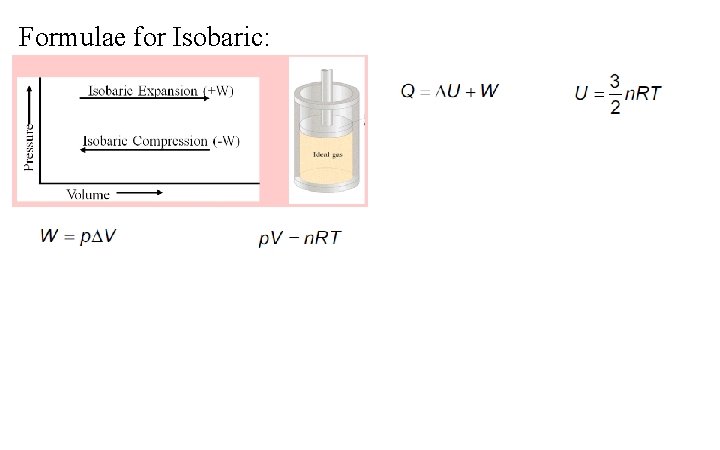
Formulae for Isobaric:
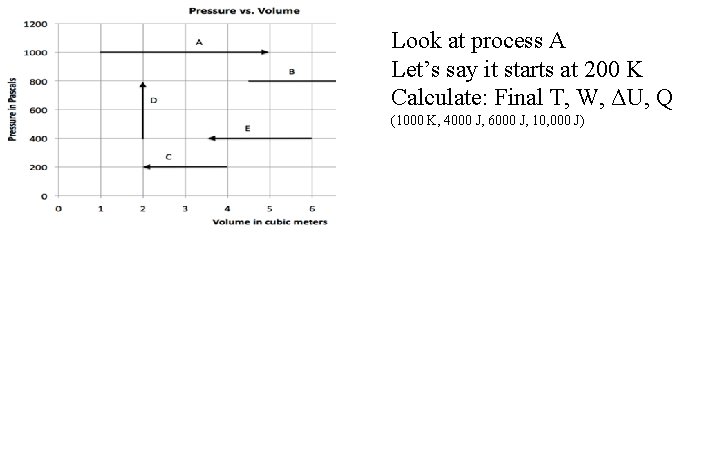
Look at process A Let’s say it starts at 200 K Calculate: Final T, W, ΔU, Q (1000 K, 4000 J, 6000 J, 10, 000 J)
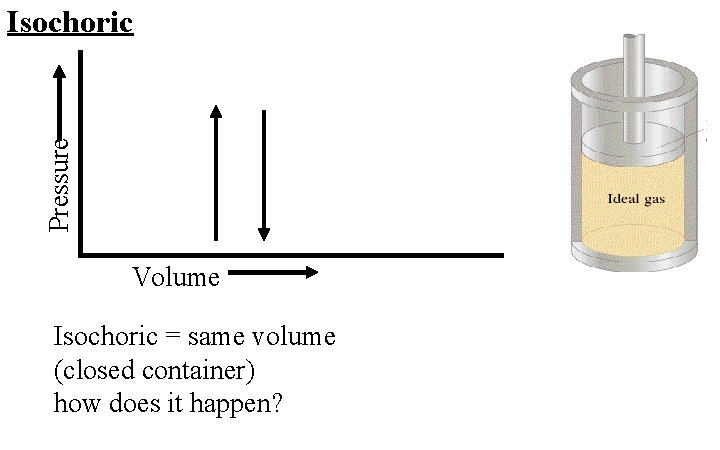
Pressure Isochoric Volume Isochoric = same volume (closed container) how does it happen?
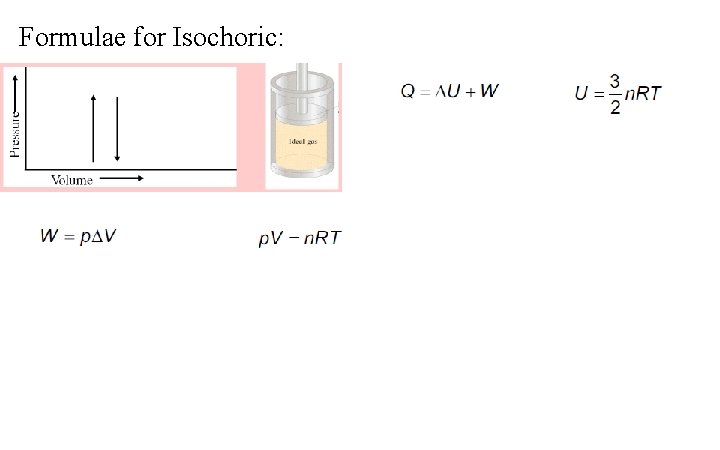
Formulae for Isochoric:
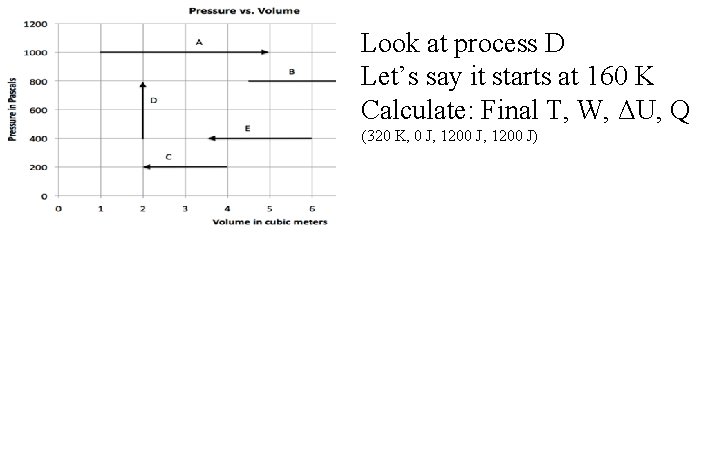
Look at process D Let’s say it starts at 160 K Calculate: Final T, W, ΔU, Q (320 K, 0 J, 1200 J)
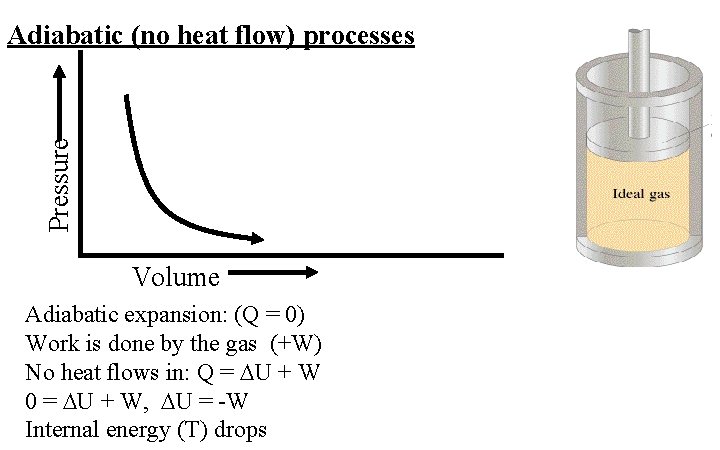
Pressure Adiabatic (no heat flow) processes Volume Adiabatic expansion: (Q = 0) Work is done by the gas (+W) No heat flows in: Q = U + W 0 = U + W, U = -W Internal energy (T) drops
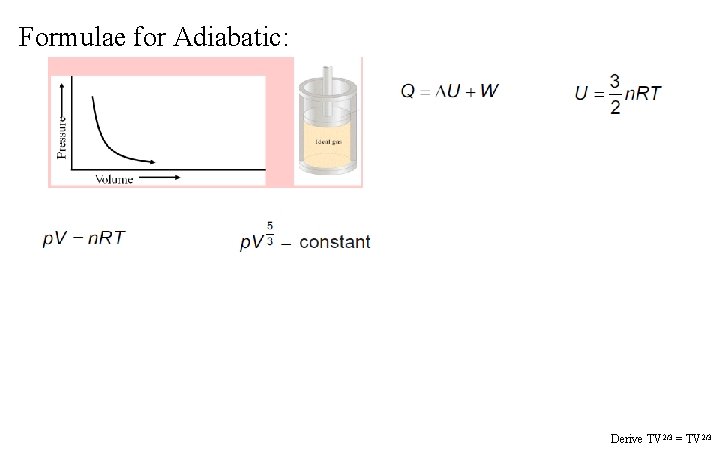
Formulae for Adiabatic: Derive TV 2/3 = TV 2/3
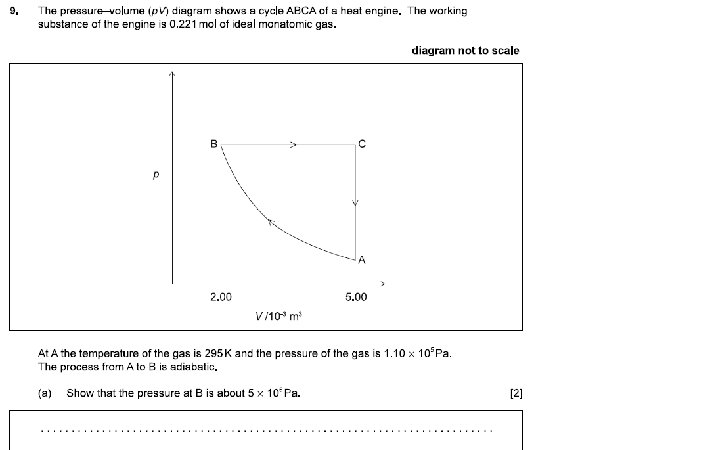
TA = 295 K, PA = 1. 10 x 105 Pa Find: n, PB, TB, and for the process AB, find Q, ΔU and W 0. 224 mol, 5. 07 E 5 Pa, 543 K, 0 J, +695 J, -695 J
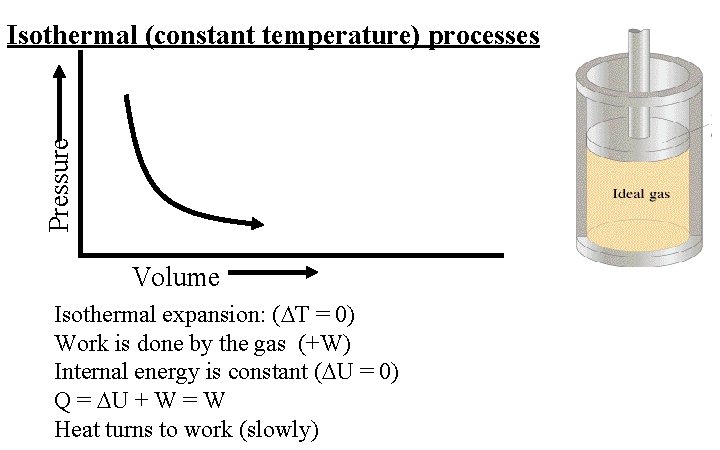
Pressure Isothermal (constant temperature) processes Volume Isothermal expansion: ( T = 0) Work is done by the gas (+W) Internal energy is constant ( U = 0) Q = U + W = W Heat turns to work (slowly)
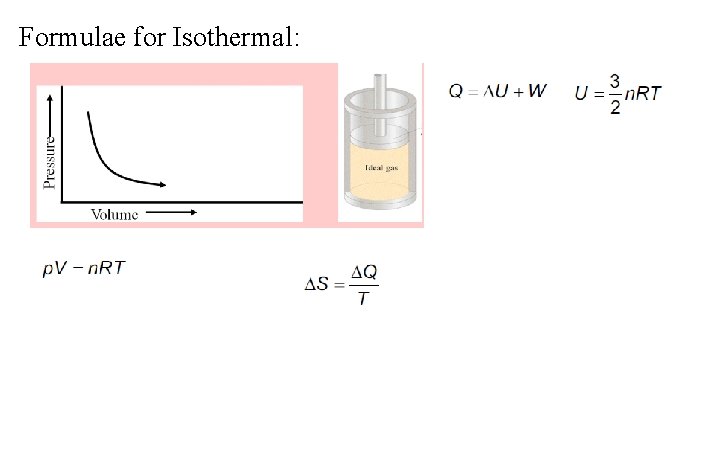
Formulae for Isothermal:
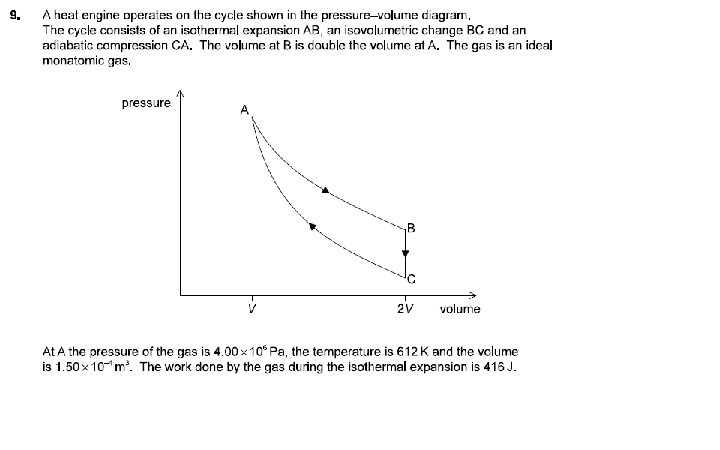
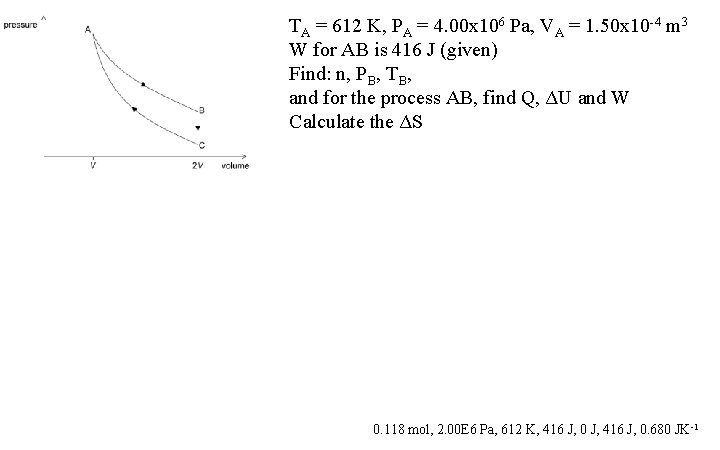
TA = 612 K, PA = 4. 00 x 106 Pa, VA = 1. 50 x 10 -4 m 3 W for AB is 416 J (given) Find: n, PB, TB, and for the process AB, find Q, ΔU and W Calculate the ΔS 0. 118 mol, 2. 00 E 6 Pa, 612 K, 416 J, 0 J, 416 J, 0. 680 JK-1
- Slides: 14