Is compound binary YES NO Is the 1

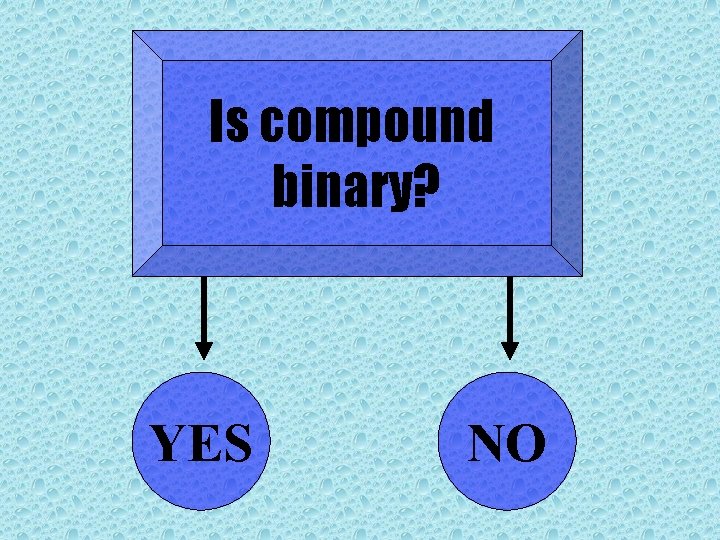
Is compound binary? YES NO
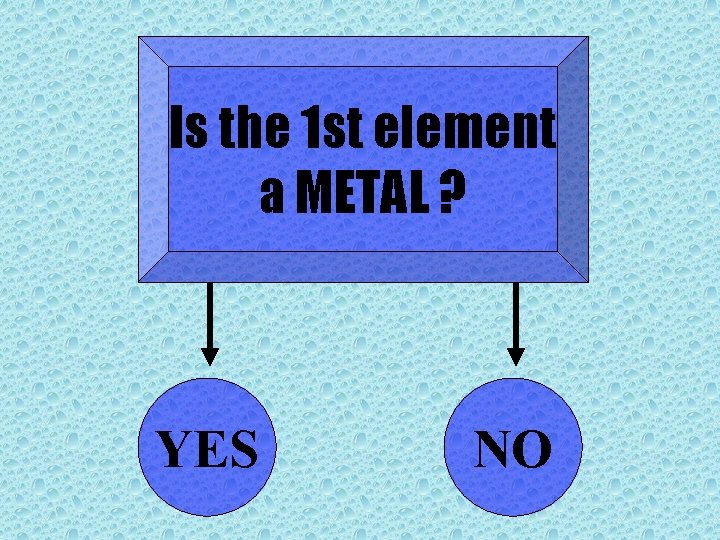
Is the 1 st element a METAL ? YES NO
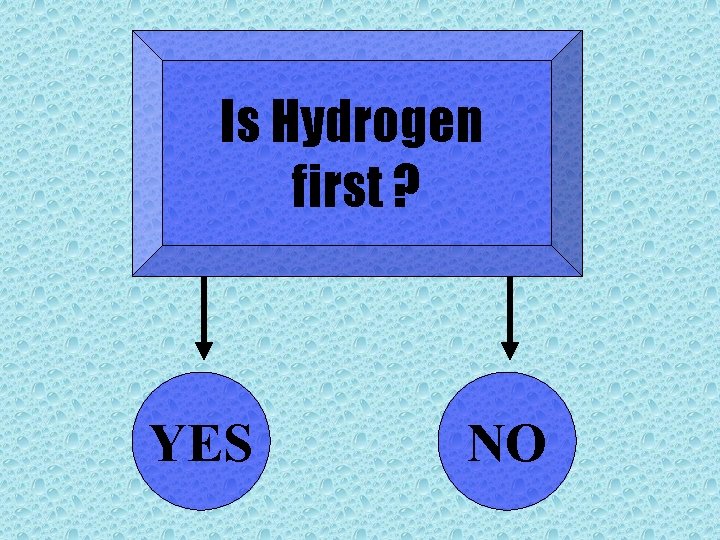
Is Hydrogen first ? YES NO
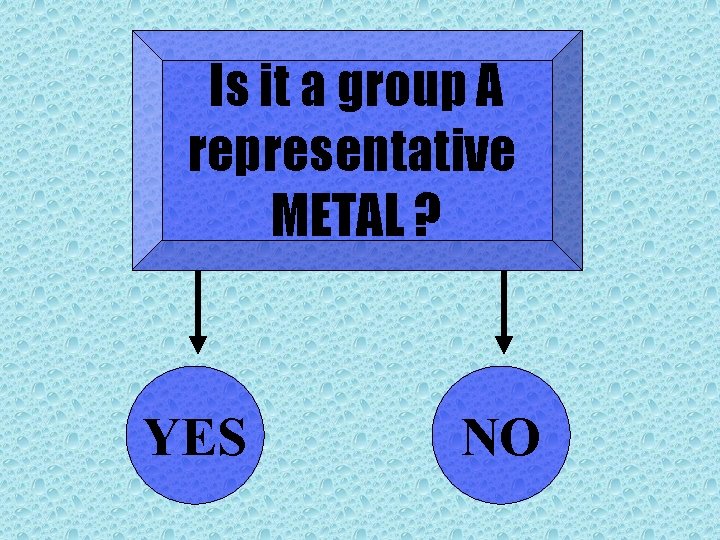
Is it a group A representative METAL ? YES NO
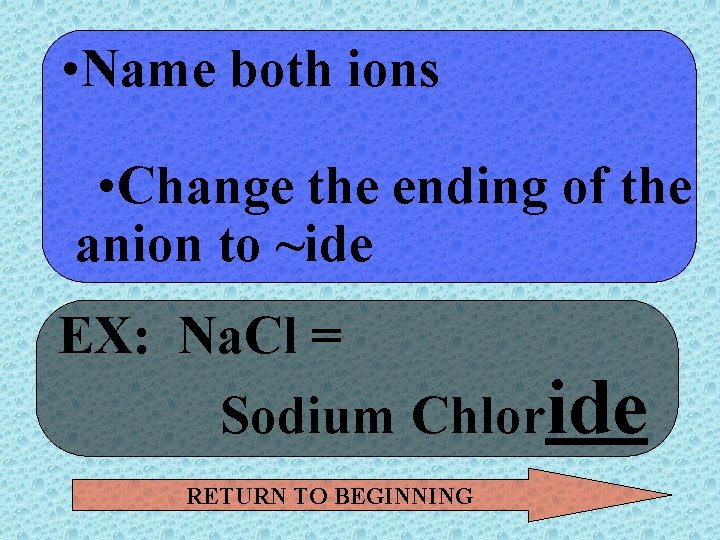
• Name both ions • Change the ending of the anion to ~ide EX: Na. Cl = Sodium Chloride RETURN TO BEGINNING
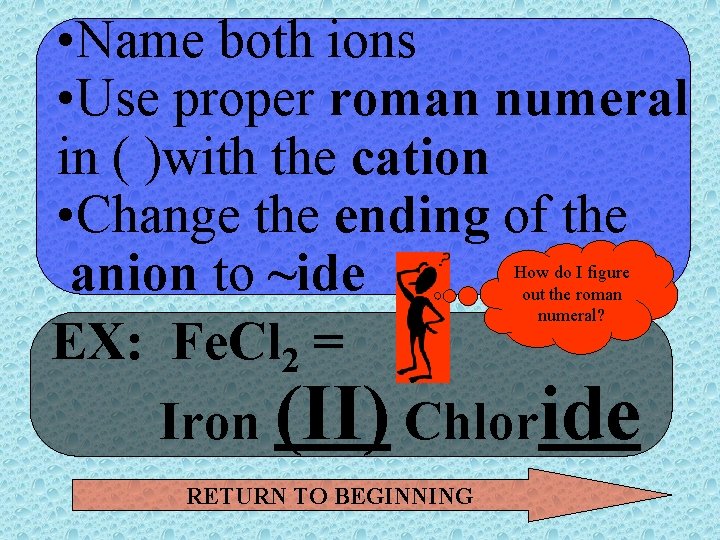
• Name both ions • Use proper roman numeral in ( )with the cation • Change the ending of the anion to ~ide EX: Fe. Cl 2 = Iron (II) Chloride How do I figure out the roman numeral? RETURN TO BEGINNING
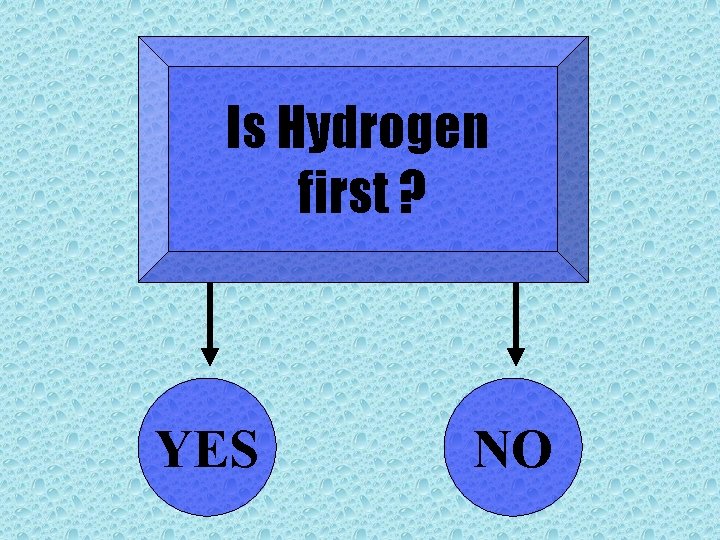
Is Hydrogen first ? YES NO
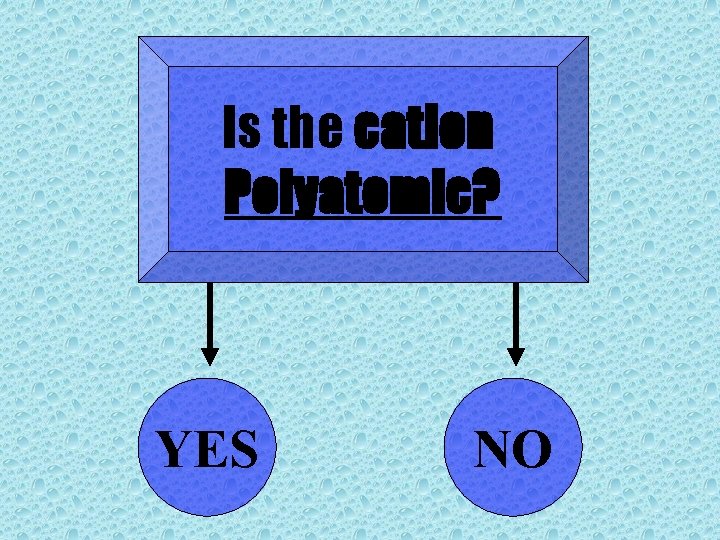
Is the cation Polyatomic? YES NO

• You have a binary acid • Add “hydro” to beginning • Change ~ide on anion to ~ic • Add “acid” to ending EX: HCl= Hydrochloric Acid RETURN TO BEGINNING
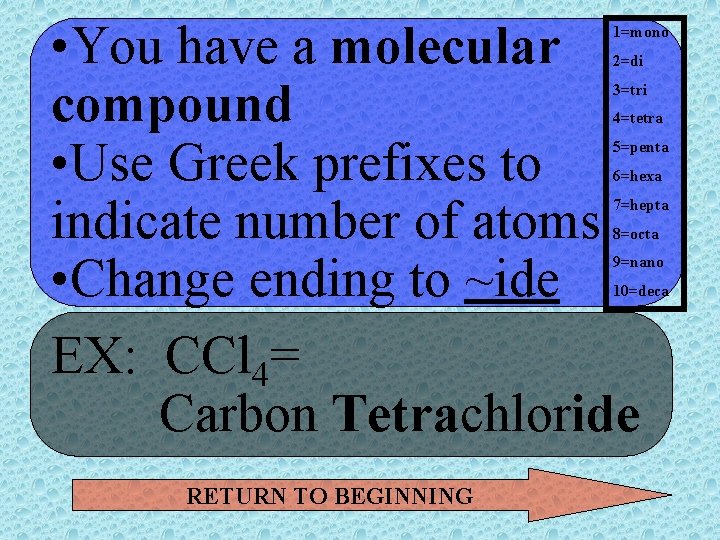
• You have a molecular compound • Use Greek prefixes to indicate number of atoms • Change ending to ~ide EX: CCl 4= Carbon Tetrachloride 1=mono 2=di 3=tri 4=tetra 5=penta 6=hexa 7=hepta 8=octa 9=nano 10=deca RETURN TO BEGINNING

ROMAN NUMERALS? ? ? • Transition metals can exist in more than one oxidation state (have more than 1 charge) To determine the charge on the ion in question: 1. Determine the charge of the ANION (look on periodic table) 2. Look at the number of anions present (written down below) 3. Determine the TOTAL negative charge 4. Look at the number of cations present 5. Divide TOTAL negative charge by number of cations present 6. This is the Roman numeral you place in ( ) after the cation name BACK TO NAME Show me an example
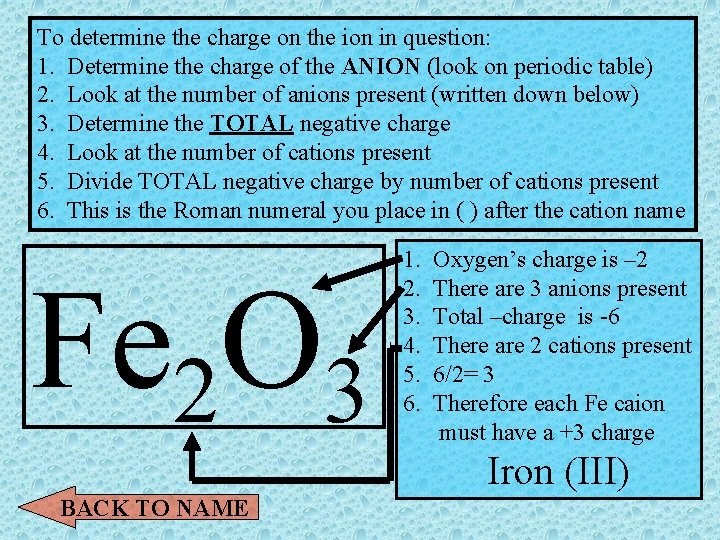
To determine the charge on the ion in question: 1. Determine the charge of the ANION (look on periodic table) 2. Look at the number of anions present (written down below) 3. Determine the TOTAL negative charge 4. Look at the number of cations present 5. Divide TOTAL negative charge by number of cations present 6. This is the Roman numeral you place in ( ) after the cation name Fe 2 O 3 1. 2. 3. 4. 5. 6. Oxygen’s charge is – 2 There are 3 anions present Total –charge is -6 There are 2 cations present 6/2= 3 Therefore each Fe caion must have a +3 charge Iron (III) BACK TO NAME

• Compound is an oxyacid • Change ~ate to ~ic ~OR~ • Change ~ite to ~ous • Add “Acid” EX: H 2 SO 4= Sulfuric Acid RETURN TO BEGINNING

• Name cation • If anion is: • single element change the ending of the anion to ~ide • also polyatomic write name of anion EX: NH 4 Cl = Ammonium Chloride EX: NH 4 NO 3=Ammonium Nitrate RETURN TO BEGINNING
Is it a group A representative METAL ? YES NO

• Name the cation • Name the anion *will end with ~ate or ~ite* (hydroxide is exception) EX: Li. CO 3 = Lithium Carbonate RETURN TO BEGINNING

• Name both ions • Use proper roman numeral in ( )with the cation • name the anion How do I figure out the roman numeral? EX: Fe 2(CO 3)3 = Iron (III) Carbonate RETURN TO BEGINNING

ROMAN NUMERALS? ? ? • Transition metals can exist in more than one oxidation state (have more than 1 charge) To determine the charge on the ion in question: 1. Determine the charge of the ANION (look on periodic table) 2. Look at the number of anions present (written down below) 3. Determine the TOTAL negative charge 4. Look at the number of cations present 5. Divide TOTAL negative charge by number of cations present 6. This is the Roman numeral you place in ( ) after the cation name BACK TO NAME Show me an example
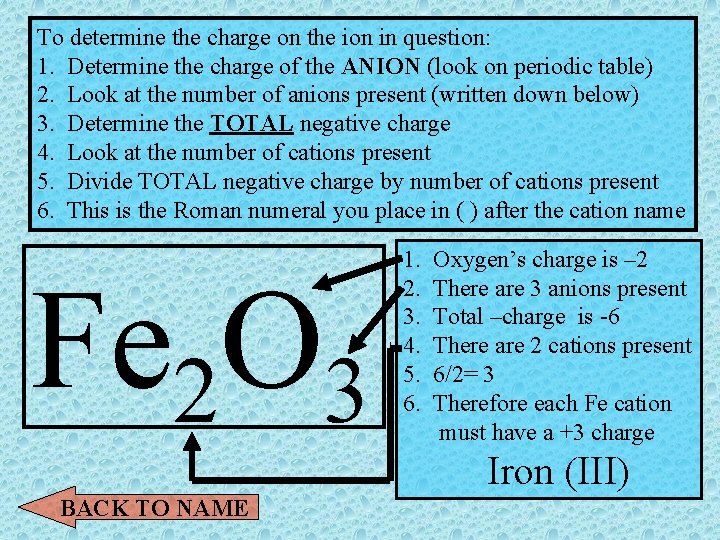
To determine the charge on the ion in question: 1. Determine the charge of the ANION (look on periodic table) 2. Look at the number of anions present (written down below) 3. Determine the TOTAL negative charge 4. Look at the number of cations present 5. Divide TOTAL negative charge by number of cations present 6. This is the Roman numeral you place in ( ) after the cation name Fe 2 O 3 1. 2. 3. 4. 5. 6. Oxygen’s charge is – 2 There are 3 anions present Total –charge is -6 There are 2 cations present 6/2= 3 Therefore each Fe cation must have a +3 charge Iron (III) BACK TO NAME
- Slides: 20