Iron Copper and Aluminium Chemistry revision In this





- Slides: 8

Iron, Copper and Aluminium Chemistry revision

In this lesson you will re-visit… • The Reactivity Series and why copper, aluminium and iron are extracted from their ores the way they are. • What processes are used to extract the metals from their ores.

You need to be able to… • Rank metals in the Reactivity Series. • State the reason why Carbon is important in the Reactivity series. • State how a metal will be extracted given it’s position in the Reactivity Series. • Label the equipment used to extract iron, aluminium and copper. • Be able to write the equations (word and formula) for these reactions. • Explain reduction and oxidation in terms of electron loss and gain (OILRIG).

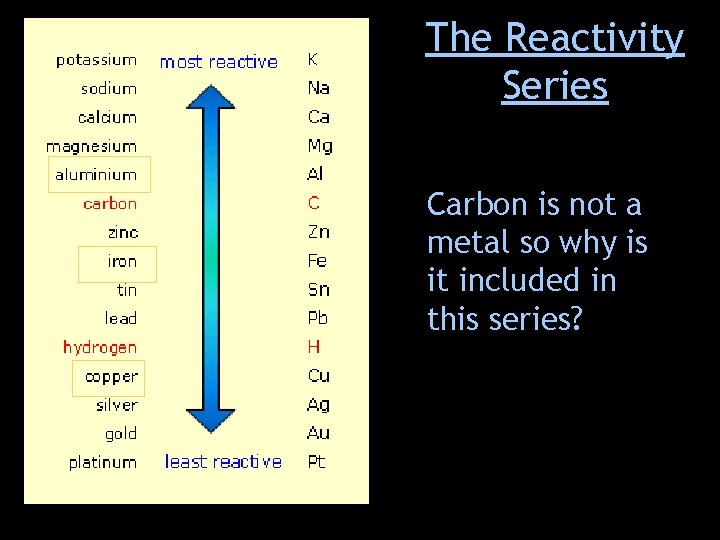
The Reactivity Series Carbon is not a metal so why is it included in this series?

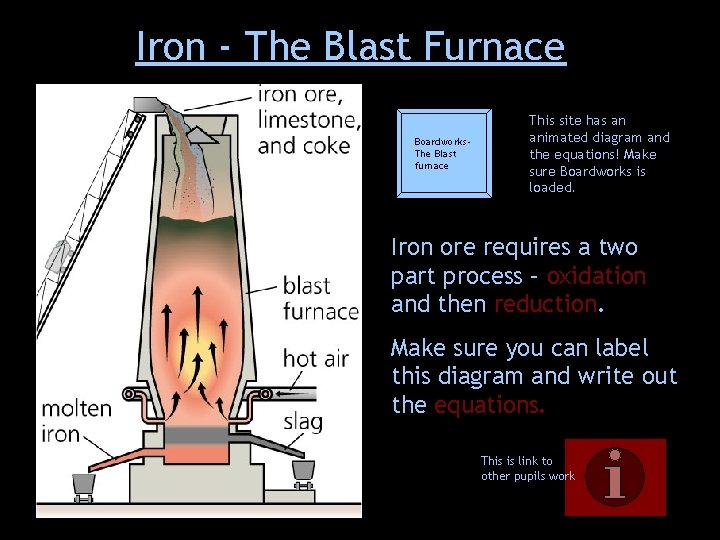
Iron - The Blast Furnace Boardworks. The Blast furnace This site has an animated diagram and the equations! Make sure Boardworks is loaded. Iron ore requires a two part process – oxidation and then reduction. Make sure you can label this diagram and write out the equations. This is link to other pupils work

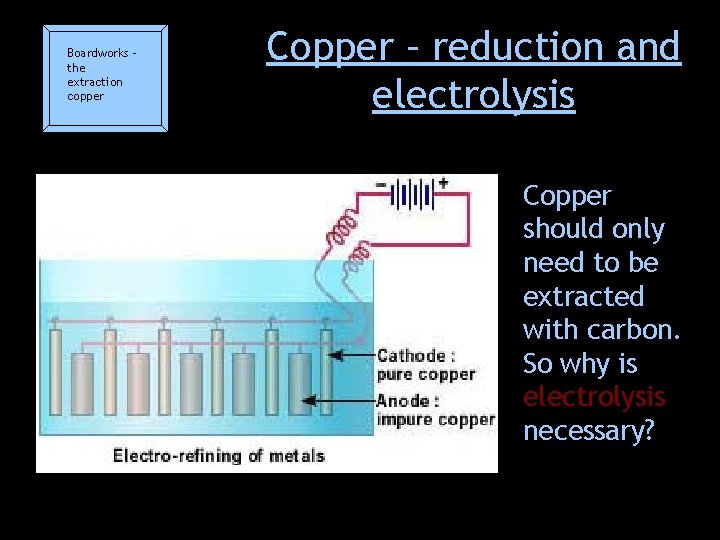
Boardworks – the extraction copper Copper – reduction and electrolysis Copper should only need to be extracted with carbon. So why is electrolysis necessary?

Aluminium – the Hall Heroult electrolysis cell Boardworks – Aluminium extraction Aluminium cannot be extracted by carbon at all. This process uses HUGE amounts of electricity. Think about environmental implications.

So can you? Name the correct order of metals in the Reactivity Series? Label the extraction processes for iron, copper and aluminium. Write out the word and symbol equations for each of the processes? Discuss the environmental implications of metal extraction processes? Interactive quiz
 Passive voice revision
Passive voice revision Mass of iron in an iron tablet
Mass of iron in an iron tablet Iron sharpens iron friendship
Iron sharpens iron friendship Aluminum sulfate + calcium hydroxide
Aluminum sulfate + calcium hydroxide Sulphuric acid and aluminium
Sulphuric acid and aluminium What is made up of silicon and aluminium
What is made up of silicon and aluminium What is made up of silicon and aluminium
What is made up of silicon and aluminium Aluminum and oxygen ionic compound formula
Aluminum and oxygen ionic compound formula Schmelzflusselektrolyse chemgapedia
Schmelzflusselektrolyse chemgapedia