Iron Carbon Phase Diagram Allotropic Transformations in Iron

Iron Carbon Phase Diagram
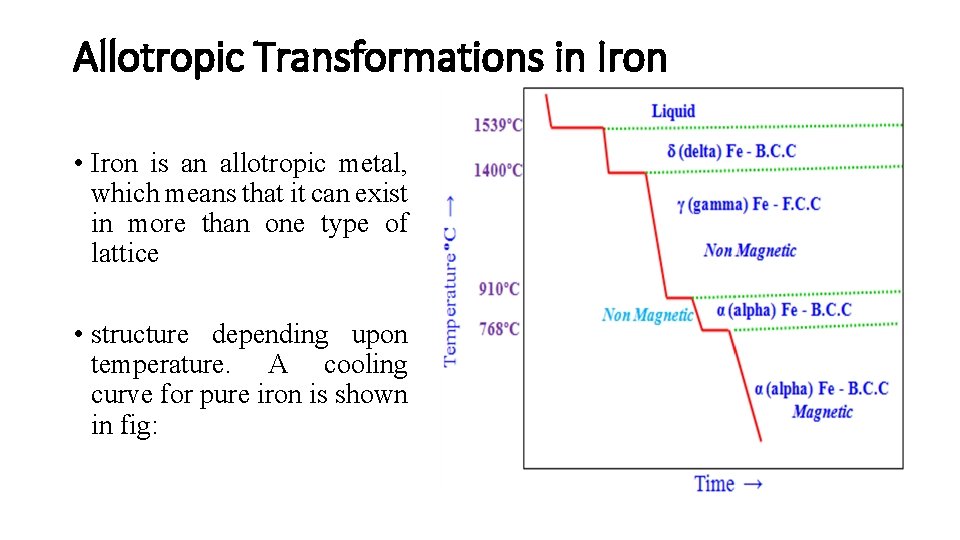
Allotropic Transformations in Iron • Iron is an allotropic metal, which means that it can exist in more than one type of lattice • structure depending upon temperature. A cooling curve for pure iron is shown in fig:
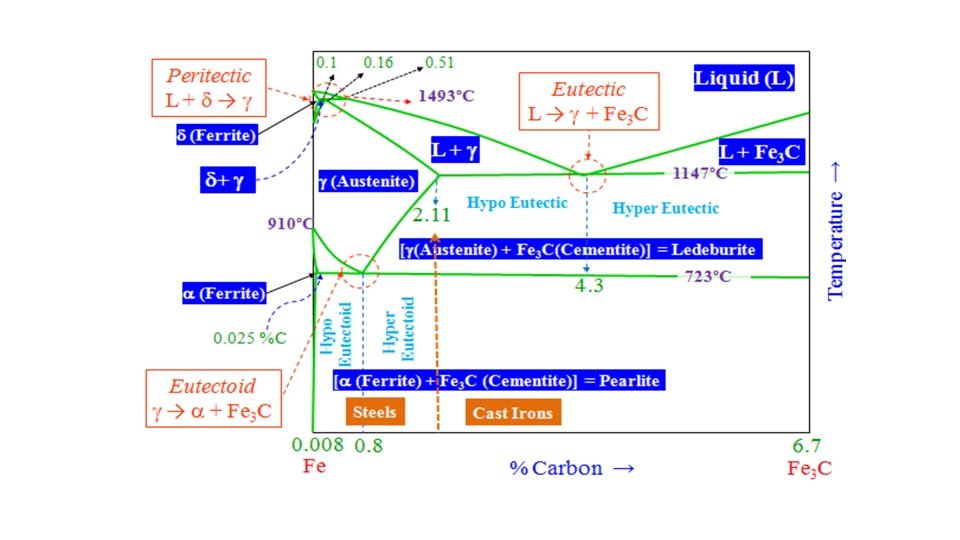
The Iron–Iron Carbide (Fe–Fe 3 C) Phase Diagram • The Fe-C (or more precisely the Fe-Fe 3 C) diagram is an important one. Cementite is a metastable phase and ‘strictly speaking’ should not be included in a phase diagram. But the decomposition rate of cementite is small and hence can be thought of as ‘stable enough’ to be included in a phase diagram. Hence, we typically consider the Fe-Fe 3 C part of the Fe-C phase diagram. • C is an interstitial impurity in Fe. It forms a solid solution with α, γ, δ phases of iron
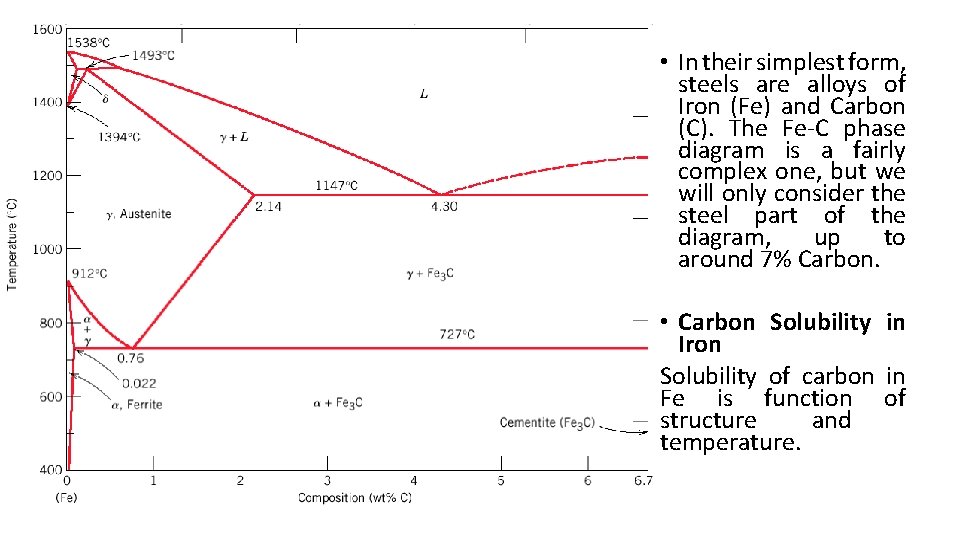
• In their simplest form, steels are alloys of Iron (Fe) and Carbon (C). The Fe-C phase diagram is a fairly complex one, but we will only consider the steel part of the diagram, up to around 7% Carbon. • Carbon Solubility in Iron Solubility of carbon in Fe is function of structure and temperature.
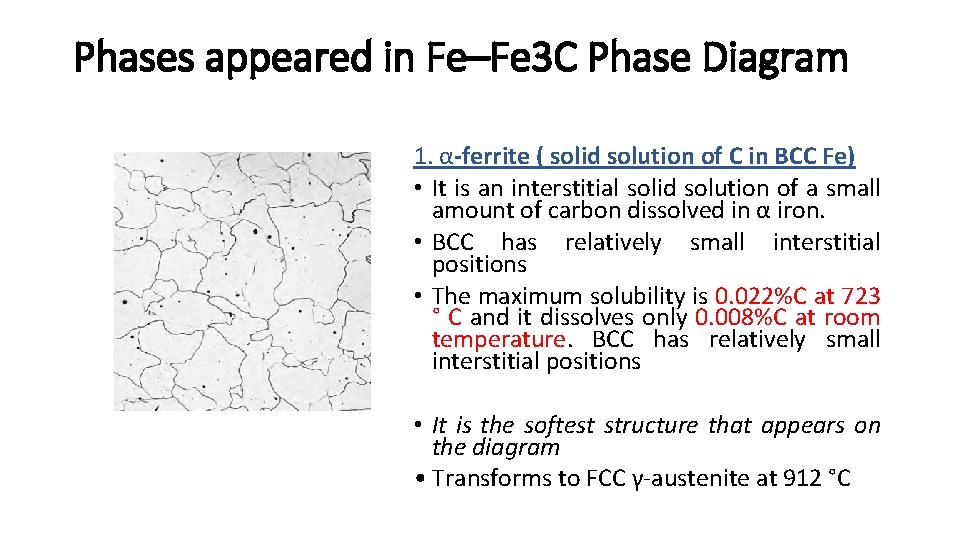
Phases appeared in Fe–Fe 3 C Phase Diagram 1. α-ferrite ( solid solution of C in BCC Fe) • It is an interstitial solid solution of a small amount of carbon dissolved in α iron. • BCC has relatively small interstitial positions • The maximum solubility is 0. 022%C at 723 ° C and it dissolves only 0. 008%C at room temperature. BCC has relatively small interstitial positions • It is the softest structure that appears on the diagram • Transforms to FCC γ-austenite at 912 °C
2. γ-austenite –(solid solution of C in FCC Fe) • The maximum solubility of C is 2. 14 wt %. at 1147 ° C. FCC has larger interstitial positions. • Transforms to BCC δ-ferrite at 1395 °C • Is not stable below the eutectic temperature(727°C) unless cooled rapidly (discuss later in unit 4)
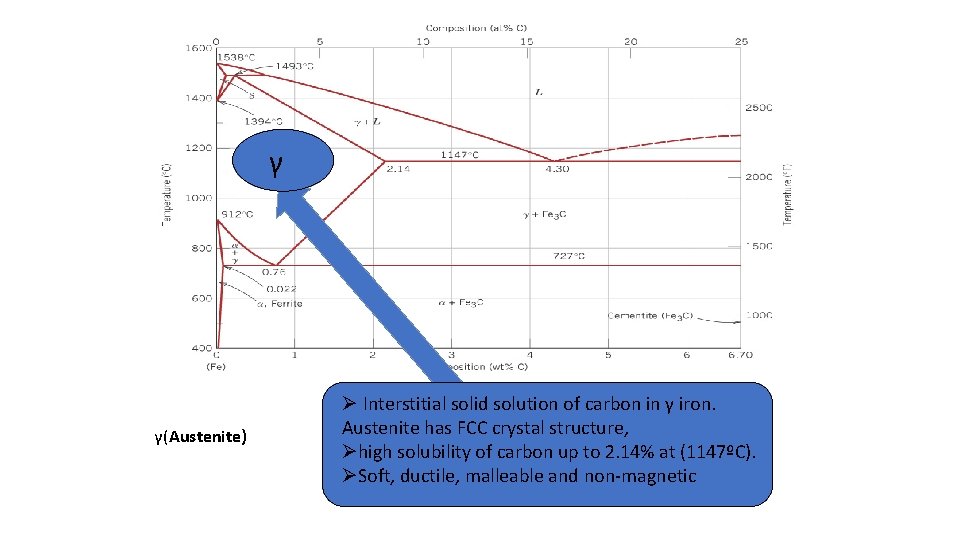
γ γ(Austenite) Ø Interstitial solid solution of carbon in γ iron. ØAustenite solid solution of carbon α-iron. has FCC crystalinstructure, ØØ α-ferrite BCC crystal structure high solubility of carbon up to 2. 14% at (1147ºC). ØØ low solubility carbon –and up to 0. 25% Soft, ductile, of malleable non-magnetic at 1333 ºF (723ºC). α-ferrite exists at RT
3. δ-ferrite (solid solution of C in BCC Fe) • The same structure as α-ferrite • Stable only at high T, above 1394 °C. The stability of the phase ranges between 1394 -1539°C. • Melts at 1538 °C 4. Fe-C liquid solution
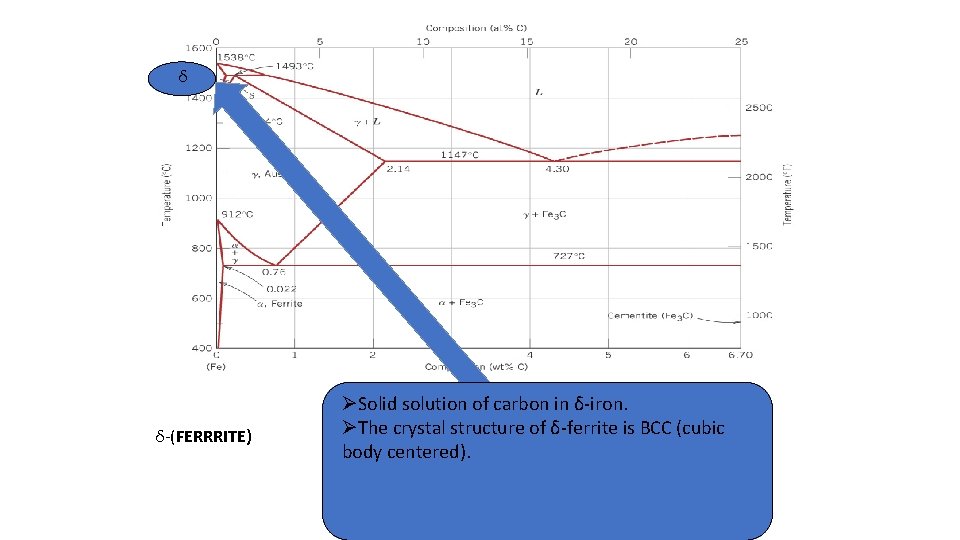
δ δ-(FERRRITE) ØSolid solution of carbon in δ-iron. ØØ solid of carbonofinδ-ferrite α-iron. is BCC (cubic The solution crystal structure Øbody α-ferrite BCC crystal structure centered). Ølow solubility of carbon – up to 0. 25% at 1333 ºF (723ºC). α-ferrite exists at RT
5. Fe 3 C (iron carbide or cementite) • This intermetallic compound is metastable, it remains as a compound indefinitely at room T, but decomposes (very slowly, within several years) into α-Fe and C (graphite) at 650 - 700 °C • It is typically hard and brittle interstitial compound of low tensile strength (approx. 5000 psi) but high compressive strength. • It is the hardest structure that appears on the diagram.
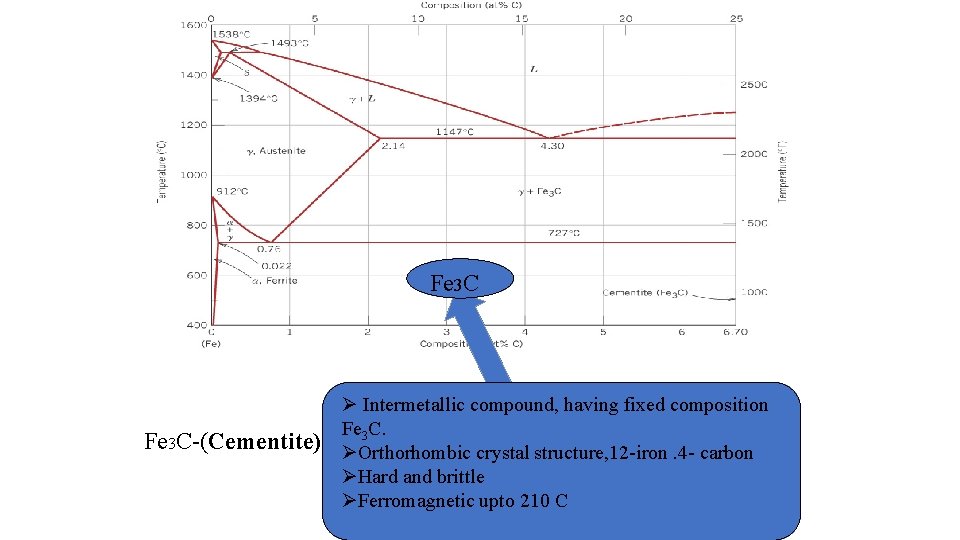
Fe 3 C Ø Intermetallic compound, having fixed composition ØFe solid solution of carbon in α-iron. C. 3 Fe 3 C-(Cementite) ØØα-ferrite BCC crystal Orthorhombic crystalstructure, 12 -iron. 4 - carbon ØØ low solubility of carbon – up to 0. 25% Hard and brittle atØ 1333 ºF (723ºC). upto α-ferrite Ferromagnetic 210 Cexists at RT
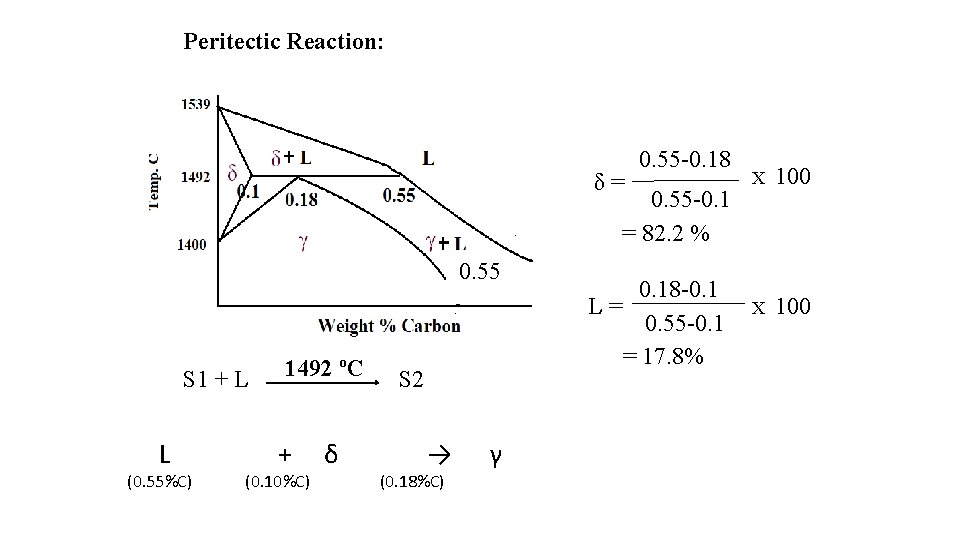
Peritectic Reaction: δ= 0. 55 -0. 18 0. 55 -0. 1 = 82. 2 % 0. 55 S 1 + L L (0. 55%C) 1492 ºC + (0. 10%C) δ S 2 → (0. 18%C) γ 0. 18 -0. 1 L= 0. 55 -0. 1 = 17. 8% X 100
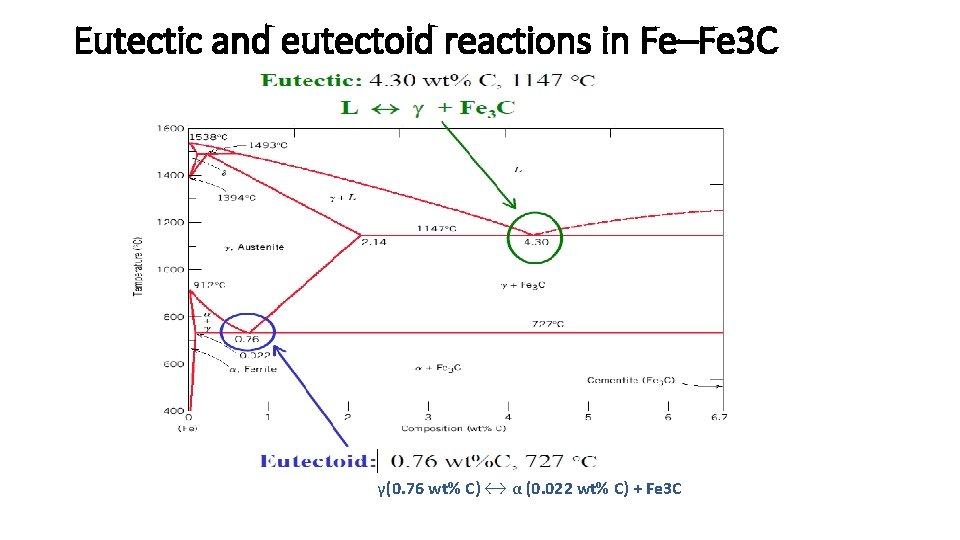
Eutectic and eutectoid reactions in Fe–Fe 3 C γ(0. 76 wt% C) ↔ α (0. 022 wt% C) + Fe 3 C
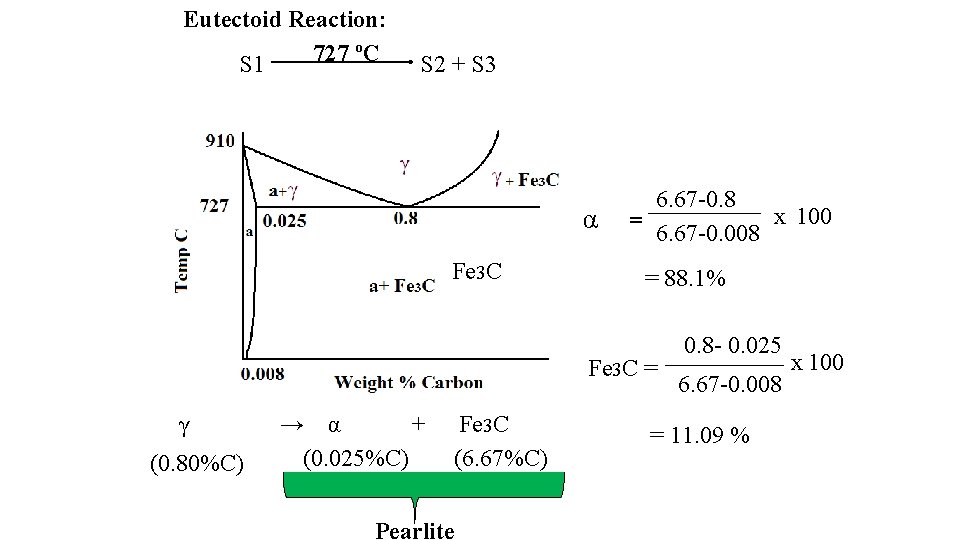
Eutectoid Reaction: 727 ºC S 1 S 2 + S 3 α Fe 3 C 6. 67 -0. 8 x 100 = 6. 67 -0. 008 = 88. 1% Fe 3 C = γ (0. 80%C) → α + (0. 025%C) Fe Fe 33 C C (6. 67%C) Pearlite 0. 8 - 0. 025 6. 67 -0. 008 = 11. 09 % x 100
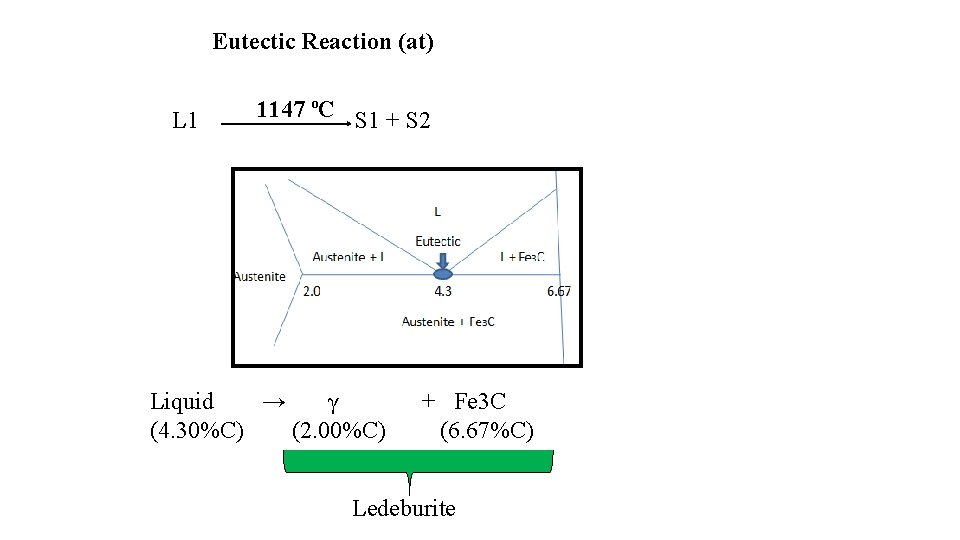
Eutectic Reaction (at) L 1 1147 ºC S 1 + S 2 Liquid → γ (4. 30%C) (2. 00%C) + Fe 3 C (6. 67%C) Ledeburite
- Slides: 16