IPS Unit 8 Periodic Table Section 2 Atomic
IPS Unit 8 Periodic Table Section 2
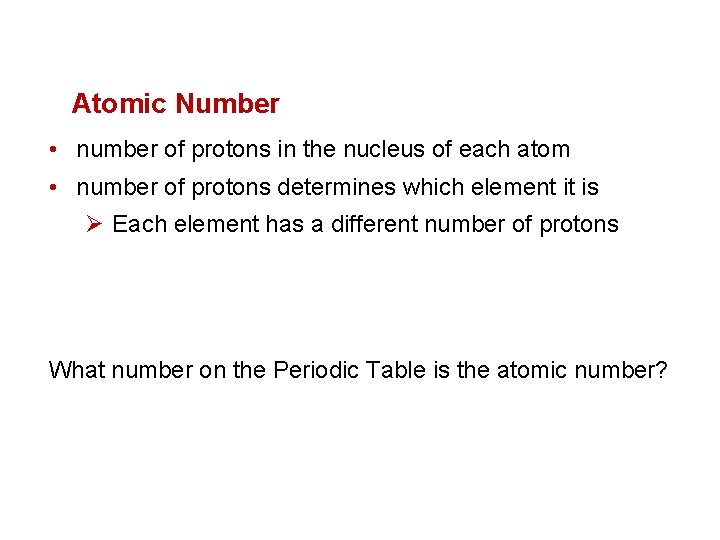
Atomic Number • number of protons in the nucleus of each atom • number of protons determines which element it is Ø Each element has a different number of protons What number on the Periodic Table is the atomic number?
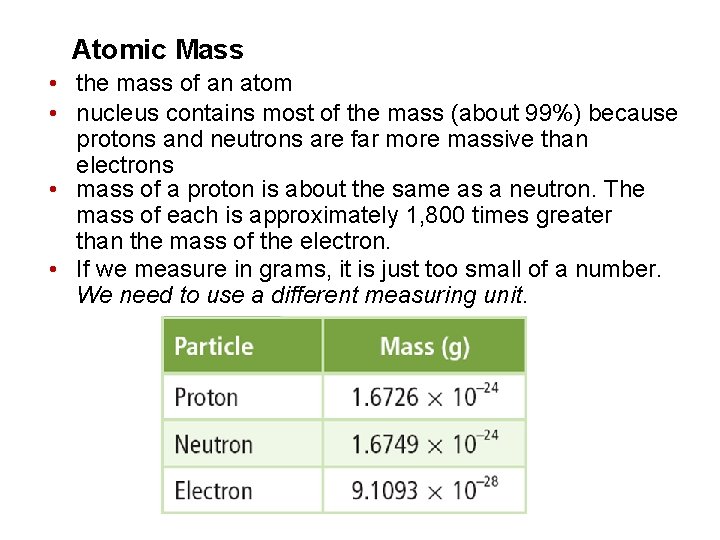
Atomic Mass • the mass of an atom • nucleus contains most of the mass (about 99%) because protons and neutrons are far more massive than electrons • mass of a proton is about the same as a neutron. The mass of each is approximately 1, 800 times greater than the mass of the electron. • If we measure in grams, it is just too small of a number. We need to use a different measuring unit.

Atomic Mass • unit of measurement for atomic particles on the Periodic Table is the atomic mass unit (amu) • mass of a proton or a neutron is equal to 1 amu • atomic mass is determined from the Carbon-12 atom Mass Number • number of protons and neutrons • this will always be a whole number (can’t have part of a proton or neutron) Ø if pulling from the Periodic Table, round to a whole number Ø if given in a problem, use that to determine other items
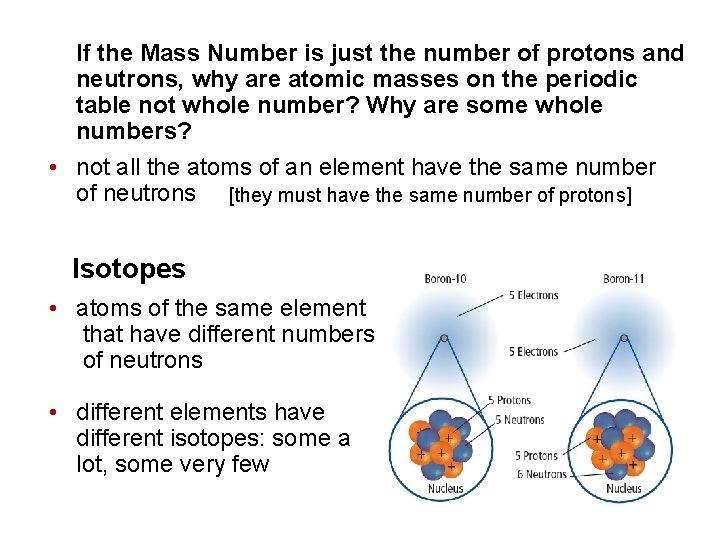
If the Mass Number is just the number of protons and neutrons, why are atomic masses on the periodic table not whole number? Why are some whole numbers? • not all the atoms of an element have the same number of neutrons [they must have the same number of protons] Isotopes • atoms of the same element that have different numbers of neutrons • different elements have different isotopes: some a lot, some very few
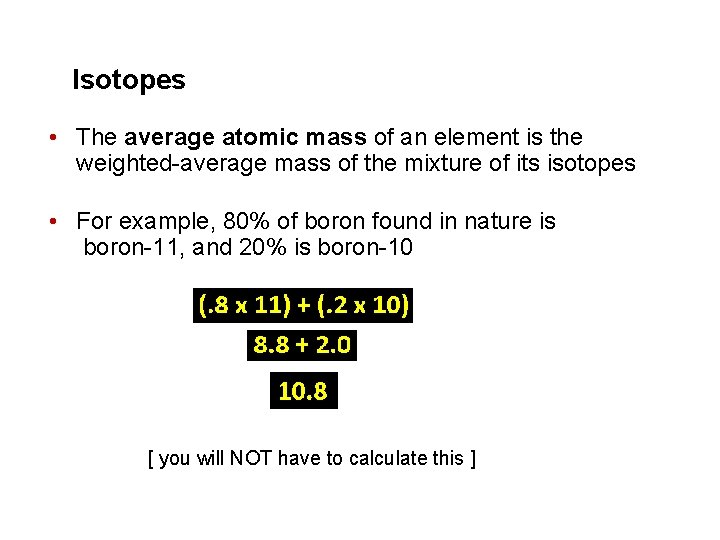
Isotopes • The average atomic mass of an element is the weighted-average mass of the mixture of its isotopes • For example, 80% of boron found in nature is boron-11, and 20% is boron-10 [ you will NOT have to calculate this ]
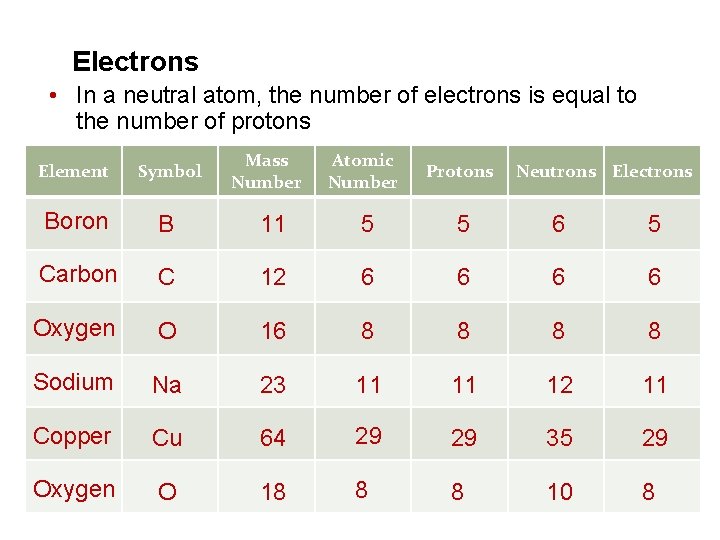
Electrons • In a neutral atom, the number of electrons is equal to the number of protons Atomic Number Protons 11 5 5 6 5 C 12 6 6 Oxygen O 16 8 8 Sodium Na 23 11 11 12 11 Copper Cu 64 29 29 35 29 Oxygen O 18 8 8 10 8 Element Symbol Boron B Carbon Mass Number Neutrons Electrons
Organizing the Elements • Periodic means "repeated in a pattern" • Dmitri Mendeleev, Russian (1860’s), searched for a way to organize the elements • arranged the elements in order of increasing atomic masses • elements with similar properties were grouped together
Organizing the Elements
Organizing the Elements • left blank spaces to keep the elements lined up according to their chemical properties • predicted properties of undiscovered elements • had some discrepancies (problems) in his table • since Mendeleev’s pattern repeated, it was considered to be periodic. Today, this arrangement is called the Periodic Table of Elements • “Father of the Periodic Table”

Changes in the Periodic Table • Henry G. J. Moseley, English (1913), arranged elements based on their increasing atomic numbers instead of an arrangement based on atomic masses • resolved discrepancies in Mendeleev’s arrangement • the current periodic table uses Moseley's arrangement of the elements
Periodic Table • arrangement of the elements in order of their atomic number so that elements with similar properties fall in the same column or group Periodic Law • when elements are arranged in order of increasing atomic number, elements with similar properties appear at regular intervals
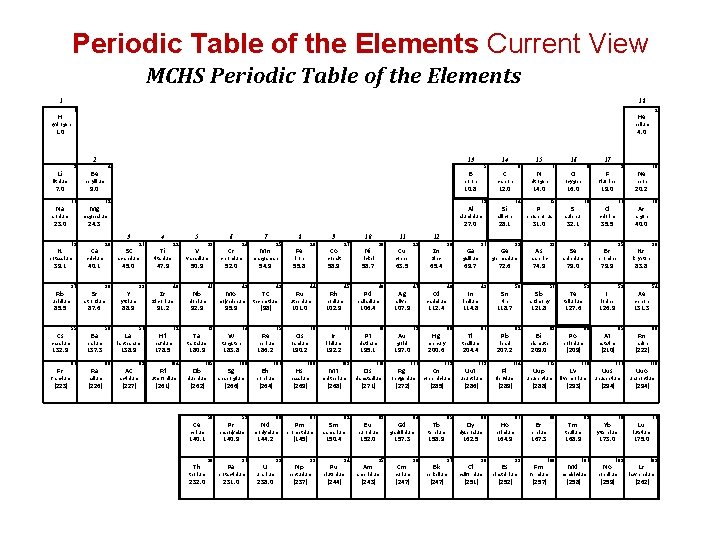
Periodic Table of the Elements Current View MCHS Periodic Table of the Elements 1 18 1 H hydrogen helium 1. 0 4. 0 2 3 Li lithium 13 4 Be boron 9. 0 11 sodium Mg aluminium 27. 0 3 K 19 potassium 39. 1 Rb 37 rubidium 55 [223] Sr Ba Ra radium [226] 21 45. 0 38 Y 39 yttrium 56 La 57 138. 9 Ac actinium [227] 22 Ti titanium 89 72 Hf hafnium 178. 5 Rf 104 rutherfordium [261] 23 V 50. 9 40 Zr 6 vanadium 47. 9 91. 2 lanthanum 88 5 zirconium 88. 9 137. 3 87 Sc scandium 40. 1 barium 132. 9 Fr calcium 87. 6 caesium francium 20 strontium 85. 5 Cs Ca 4 41 Nb niobium 73 105 Sg 58 cerium 59 praseodymium 140. 9 90 Pa 91 protactinium 231. 0 25 Mn manganese 54. 9 43 Tc technetium [98] 75 Re Bh 107 60 144. 2 238. 0 92 27 Co 44 Ru 101. 0 76 Os Pm 77 Ir 93 neptunium 62 samarium 150. 4 Pu 94 plutonium [244] Ds zinc 110 [271] Eu 63 europium 79 Au Rg 95 Si silicon phosphorus 28. 1 O 31. 0 sulphur 20. 2 17 Cl chlorine 32. 1 10 Ne neon 19. 0 16 S 9 F fluorine 16. 0 15 P 17 8 oxygen 14. 0 14 [272] Cm [247] thallium 64 96 Cn 112 copernicium [285] Tb 65 terbium 18 Ar argon 35. 5 40. 0 113 [286] Dy 66 162. 5 97 berkelium [247] Uut ununtrium Cf 98 californium [251] Fl 114 115 67 68 Er erbium 164. 9 167. 3 99 einsteinium Fm 100 fermium [257] Lv 116 [293] 69 thulium 168. 9 Md 101 mendelevium [258] Uus [222] 117 unuseptium [294] 70 Yb ytterbium 173. 0 No 102 nobelium [259] 86 Rn radon [210] livermorium Tm 131. 3 85 At 54 Xe xenon astatine [209] [288] holmium [252] Uup 84 Po 53 I 126. 9 polonium unupentium [289] Es 83 Bi 83. 8 iodine 127. 6 209. 0 flerovium Ho tellurium 36 Kr krypton 79. 9 52 Te 35 Br bromine 79. 0 51 Sb 34 Se selenium 74. 9 bismuth 207. 2 dysprosium 158. 9 Bk lead 204. 4 arsenic 121. 8 82 Pb 33 As antimony 118. 7 81 Tl 50 Sn tin 114. 8 80 Hg 49 In 32 Ge 72. 6 indium 200. 6 111 Gd 48 Cd mercury roentgenium curium N nitrogen germanium 69. 7 112. 4 157. 3 americium [243] 107. 9 31 Ga gallium cadmium gadolinium 152. 0 Am 47 Ag silver 197. 0 darmstadtium 30 Zn 65. 4 gold 195. 1 109 Sm 78 Pt platinum [268] 61 46 106. 4 meitnerium [145] [237] 102. 9 29 Cu 63. 5 Pd 16 7 12 copper palladium iridium promethium Np 45 Rh rhodium Mt 28 Ni 58. 7 192. 2 108 11 nickel 58. 9 ruthenium Hs 10 cobalt [269] neodymium uranium iron 55. 8 hassium [264] U 26 Fe 190. 2 bohrium Nd 9 osmium 186. 2 106 Pr 8 rhenium [266] 140. 1 232. 0 74 W seaborgium [262] thorium 42 Mo 183. 8 dubnium Th 52. 0 tungsten 180. 9 Ce chromium 95. 9 tantalum Db 24 Cr molybdenum 92. 9 Ta 7 C 12. 0 13 Al 24. 3 15 6 carbon 10. 8 12 magnesium 23. 0 14 5 B beryllium 7. 0 Na 2 He Uuo 118 ununoctium [294] 71 Lu lutetium 175. 0 Lr 103 lawrencium [262]
- Slides: 13