IPS Unit 12 Stoichiometry Section 3 Stoichiometry Unit





- Slides: 10

IPS Unit 12 Stoichiometry Section 3 Stoichiometry

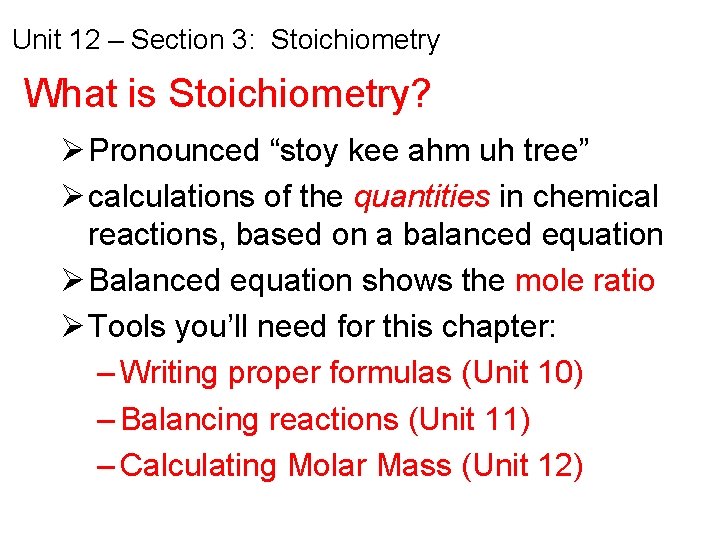
Unit 12 – Section 3: Stoichiometry What is Stoichiometry? Ø Pronounced “stoy kee ahm uh tree” Ø calculations of the quantities in chemical reactions, based on a balanced equation Ø Balanced equation shows the mole ratio Ø Tools you’ll need for this chapter: – Writing proper formulas (Unit 10) – Balancing reactions (Unit 11) – Calculating Molar Mass (Unit 12)

Unit 12 – Section 3: Stoichiometry What is Stoichiometry? Ø When calculating stoichiometry problems Everything must go through MOLES!!

Unit 12 – Section 3: Stoichiometry General Steps to Solving Stoichiometry Problems 1. Identify what you know and what you need to find. 2. Start with what you are given. 3. Use the units to guide you through the problem. 4. Check to make sure you found the answer to the question asked. *You need a balanced equation and you will need to calculate formula masses.

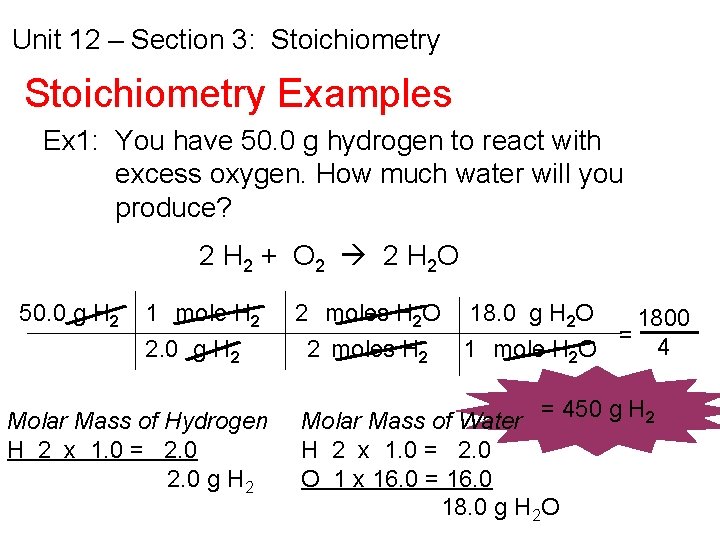
Unit 12 – Section 3: Stoichiometry Examples Ex 1: You have 50. 0 g hydrogen to react with excess oxygen. How much water will you produce? 2 H 2 + O 2 2 H 2 O 50. 0 g H 2 1 mole H 2 2. 0 g H 2 Molar Mass of Hydrogen H 2 x 1. 0 = 2. 0 g H 2 2 moles H 2 O 18. 0 g H 2 O 1800 = 4 2 moles H 2 1 mole H 2 O Molar Mass of Water = 450 g H 2 x 1. 0 = 2. 0 O 1 x 16. 0 = 16. 0 18. 0 g H 2 O

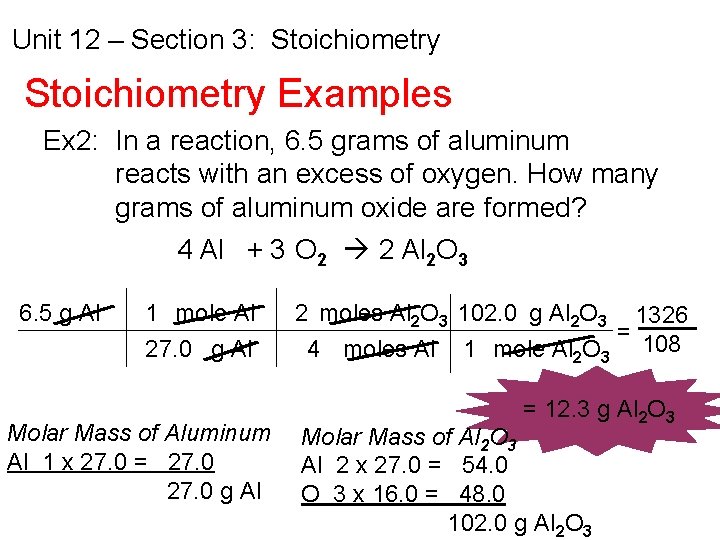
Unit 12 – Section 3: Stoichiometry Examples Ex 2: In a reaction, 6. 5 grams of aluminum reacts with an excess of oxygen. How many grams of aluminum oxide are formed? 4 Al + 3 O 2 2 Al 2 O 3 6. 5 g Al 1 mole Al 27. 0 g Al Molar Mass of Aluminum Al 1 x 27. 0 = 27. 0 g Al 2 moles Al 2 O 3 102. 0 g Al 2 O 3 1326 = 4 moles Al 1 mole Al 2 O 3 108 = 12. 3 g Al 2 O 3 Molar Mass of Al 2 O 3 Al 2 x 27. 0 = 54. 0 O 3 x 16. 0 = 48. 0 102. 0 g Al 2 O 3

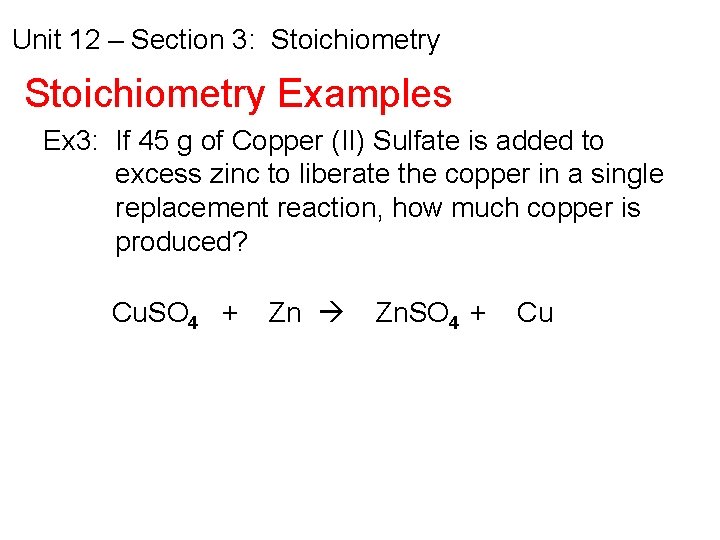
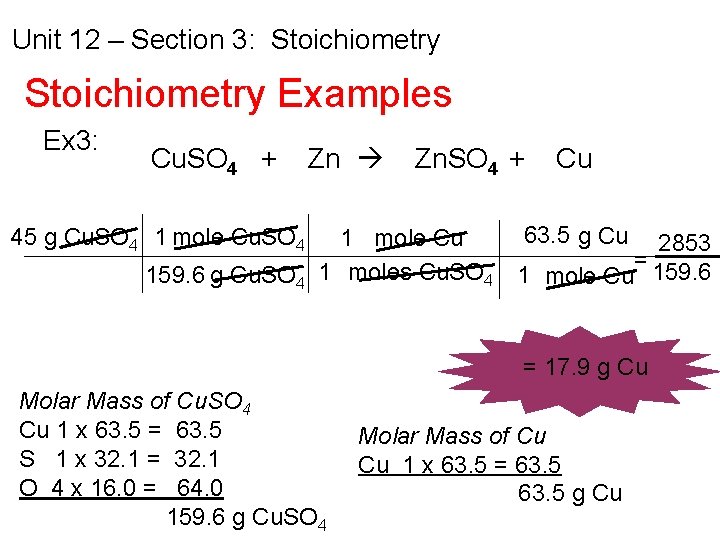
Unit 12 – Section 3: Stoichiometry Examples Ex 3: If 45 g of Copper (II) Sulfate is added to excess zinc to liberate the copper in a single replacement reaction, how much copper is produced? Cu. SO 4 + Zn Zn. SO 4 + Cu

Unit 12 – Section 3: Stoichiometry Examples Ex 3: Cu. SO 4 + Zn 45 g Cu. SO 4 1 mole Cu. SO 4 Zn. SO 4 + 1 mole Cu 159. 6 g Cu. SO 4 1 moles Cu. SO 4 Cu 63. 5 g Cu 2853 = 1 mole Cu 159. 6 = 17. 9 g Cu Molar Mass of Cu. SO 4 Cu 1 x 63. 5 = 63. 5 S 1 x 32. 1 = 32. 1 O 4 x 16. 0 = 64. 0 159. 6 g Cu. SO 4 Molar Mass of Cu Cu 1 x 63. 5 = 63. 5 g Cu

Unit 12 – Section 3: Stoichiometry Examples Ex 4: If you decompose Sodium Chloride into it elements, how much of each product do you make if you start with 30 g of reactant?

Unit 12 – Section 3: Stoichiometry Hints Ø Always start with what you are given Ø If you are given grams, convert it to moles Ø For molar mass, there is only 1 mole for X number of grams Ø Make sure you have a balanced equation to start with Ø Let the units guide you through the problem