IPC Notes Heat Transfer Conduction the transfer of













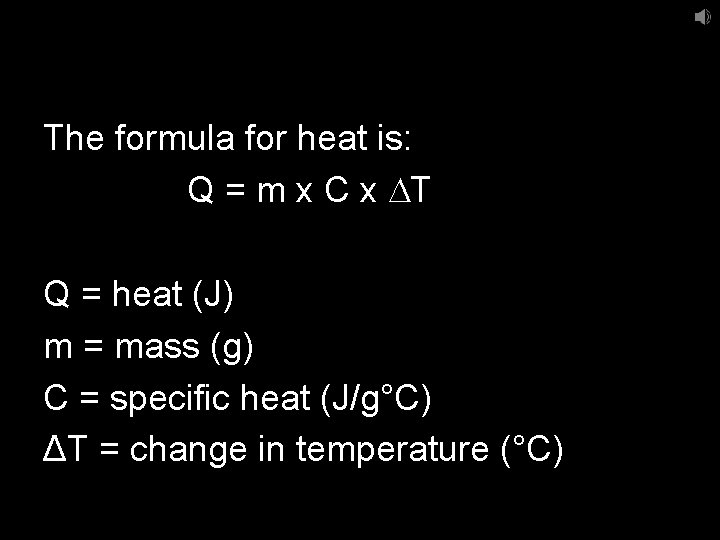
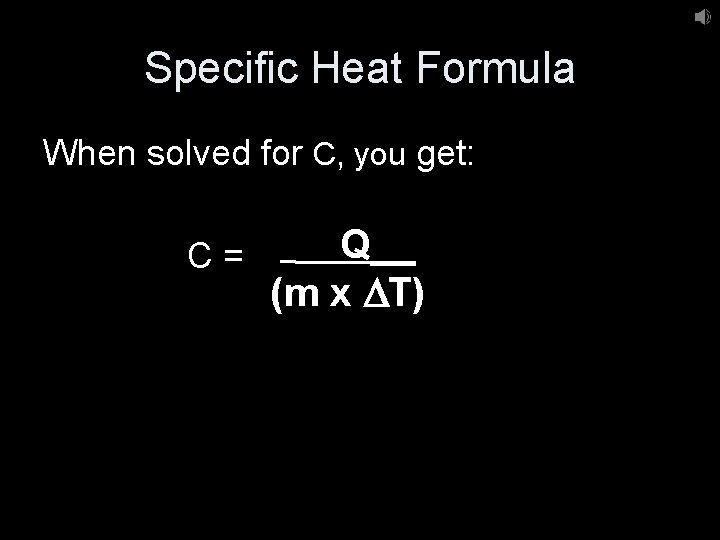

- Slides: 26

IPC Notes: Heat Transfer

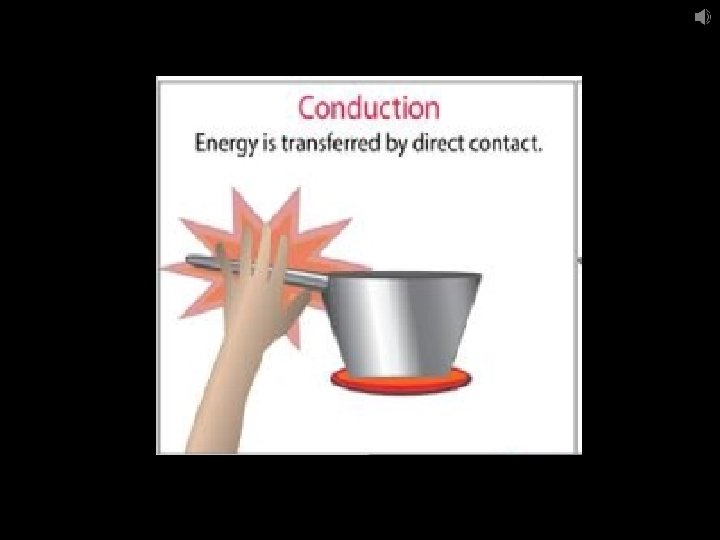
Conduction – the transfer of heat by direct contact of particles


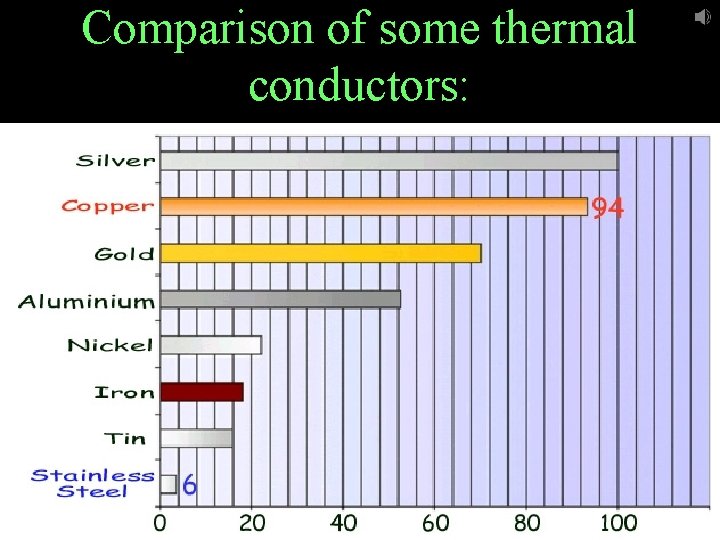
Thermal conductors – materials that conduct heat easily ex) metals

Comparison of some thermal conductors:

Thermal insulators – materials that conduct heat poorly ex) plastic, wood, rubber & glass

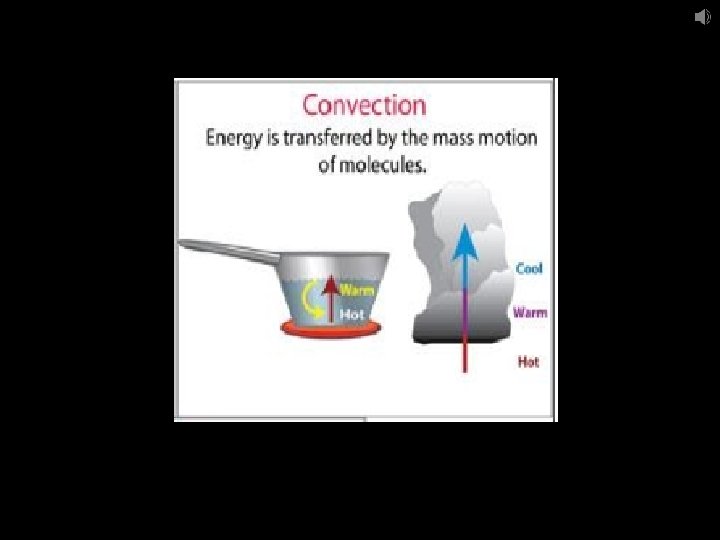
Convection – transfer of heat by the actual motion of a fluid in the form of currents warm fluids rise & cooler fluids sink



Radiation – transfer of heat by electromagnetic waves


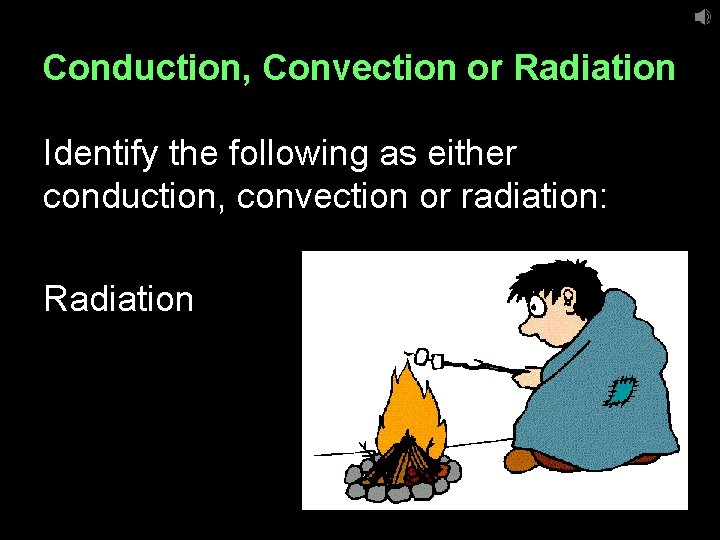
Conduction, Convection or Radiation Identify the following as either conduction, convection or radiation: The sun warms your skin Radiation

Conduction, Convection or Radiation Identify the following as either conduction, convection or radiation: A cold front makes the outside temperature drop Convection

Conduction, Convection or Radiation Identify the following as either conduction, convection or radiation: Warm air circulates inside a house during the winter Convection

Conduction, Convection or Radiation Identify the following as either conduction, convection or radiation: A child burns his hand after touching a hot stove Conduction

Conduction, Convection or Radiation Identify the following as either conduction, convection or radiation: A seatbelt burns your hand on a hot summer day Conduction

Conduction, Convection or Radiation Identify the following as either conduction, convection or radiation: Radiation

https: //www. youtube. com/watch? v=679 Gjn. LZp 2 E

Thermal energy is energy in the form of heat. Temperature is the measure of the average kinetic energy of the particles in a sample of matter.

Heat is the energy that transfers due to a difference in temperature. Heat always transfers from hot materials to cold materials.

Specific heat is the amount of energy required to raise the temperature of 1 g of substance by 1 o. C.
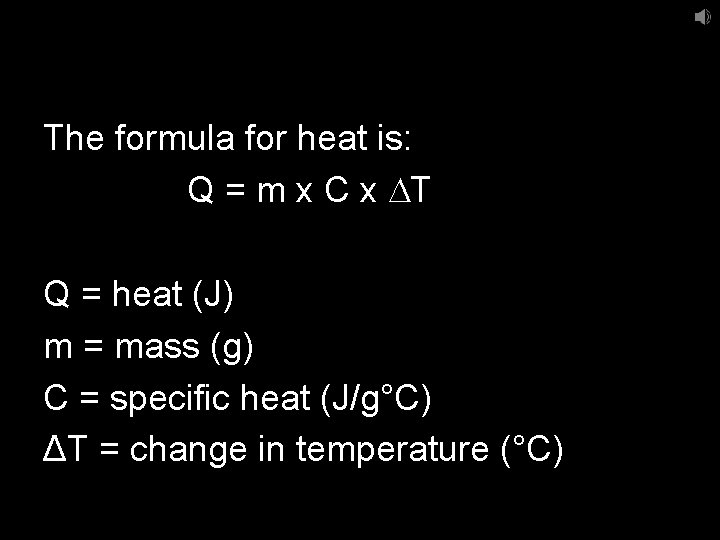
The formula for heat is: Q = m x C x DT Q = heat (J) m = mass (g) C = specific heat (J/g°C) ΔT = change in temperature (°C)
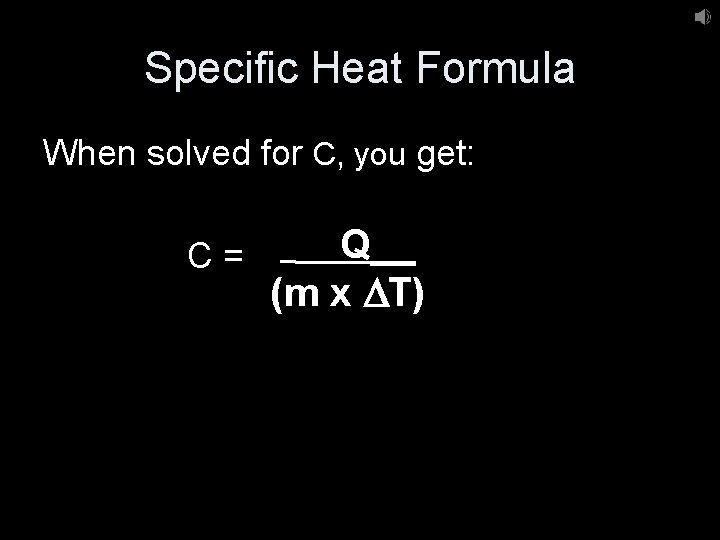
Specific Heat Formula When solved for C, you get: C= Q__ (m x DT)

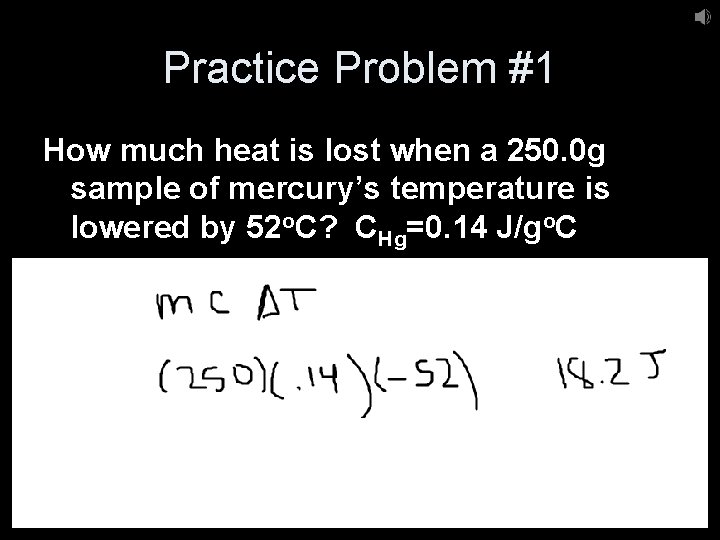
Practice Problem #1 How much heat is lost when a 250. 0 g sample of mercury’s temperature is lowered by 52 o. C? CHg=0. 14 J/go. C

Practice Problem #2 When 435 J of heat is added to 3. 4 g of olive oil, it raises the temperature from 21 o. C to 85 o. C. What is the specific heat of olive oil?

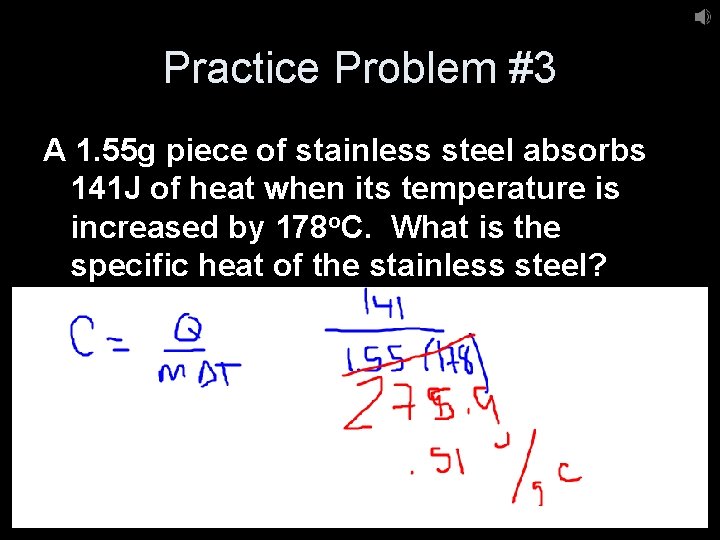
Practice Problem #3 A 1. 55 g piece of stainless steel absorbs 141 J of heat when its temperature is increased by 178 o. C. What is the specific heat of the stainless steel?