IP Valuation Dr Rahul Verma Assistant Vice President







- Slides: 36

IP Valuation Dr. Rahul Verma, Assistant Vice President, Intellectual Property and Legal Support Services Rahul. Verma@Evalueserve. com April 2010 © 2010 Evalueserve, Ltd. All Rights Reserved - Privileged and Confidential

Presentation Plan IP Valuation Overview IP Valuation Approaches Case Study Appendix Slide 2 © 2010 Evalueserve, Ltd. All Rights Reserved - Privileged and Confidential

Presentation Plan IP Valuation Overview IP Valuation Approaches Case Study Appendix Slide 3 © 2010 Evalueserve, Ltd. All Rights Reserved - Privileged and Confidential

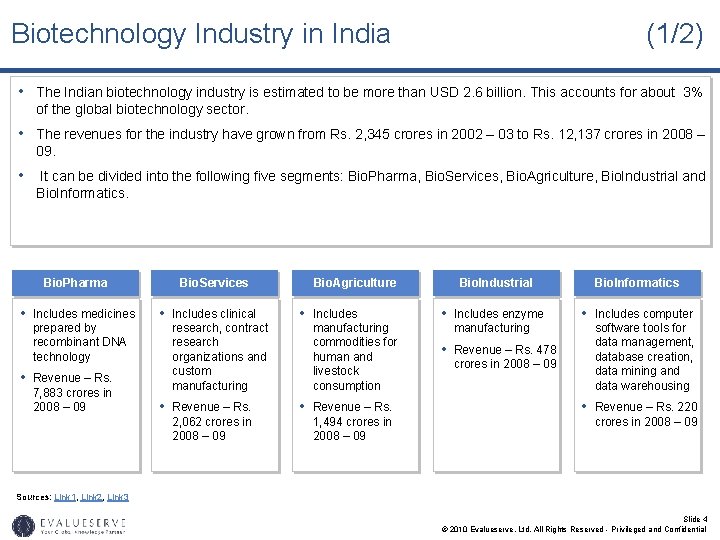
Biotechnology Industry in India (1/2) • The Indian biotechnology industry is estimated to be more than USD 2. 6 billion. This accounts for about 3% of the global biotechnology sector. • The revenues for the industry have grown from Rs. 2, 345 crores in 2002 – 03 to Rs. 12, 137 crores in 2008 – 09. • It can be divided into the following five segments: Bio. Pharma, Bio. Services, Bio. Agriculture, Bio. Industrial and Bio. Informatics. Bio. Pharma • Includes medicines prepared by recombinant DNA technology • Revenue – Rs. 7, 883 crores in 2008 – 09 Bio. Services • Includes clinical research, contract research organizations and custom manufacturing Bio. Agriculture • Includes manufacturing commodities for human and livestock consumption Bio. Industrial Bio. Informatics • Includes enzyme • Includes computer manufacturing • Revenue – Rs. 478 crores in 2008 – 09 software tools for data management, database creation, data mining and data warehousing • Revenue – Rs. 220 2, 062 crores in 2008 – 09 1, 494 crores in 2008 – 09 Sources: Link 1, Link 2, Link 3 Slide 4 © 2010 Evalueserve, Ltd. All Rights Reserved - Privileged and Confidential

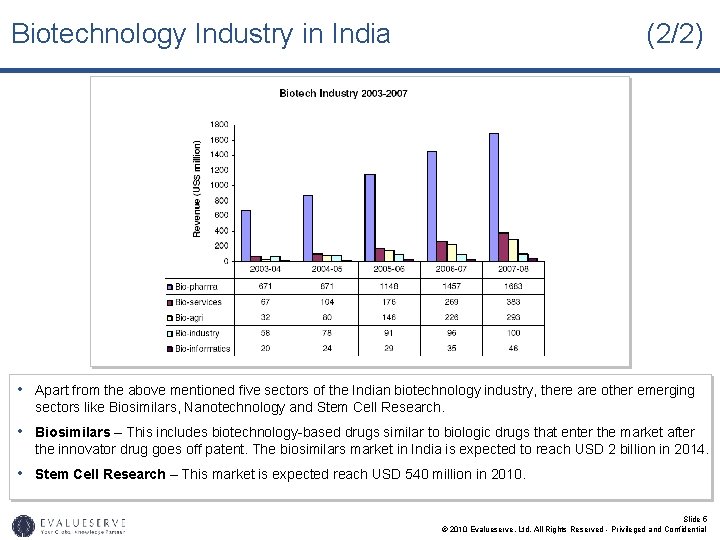
Biotechnology Industry in India (2/2) • Apart from the above mentioned five sectors of the Indian biotechnology industry, there are other emerging sectors like Biosimilars, Nanotechnology and Stem Cell Research. • Biosimilars – This includes biotechnology-based drugs similar to biologic drugs that enter the market after the innovator drug goes off patent. The biosimilars market in India is expected to reach USD 2 billion in 2014. • Stem Cell Research – This market is expected reach USD 540 million in 2010. Slide 5 © 2010 Evalueserve, Ltd. All Rights Reserved - Privileged and Confidential

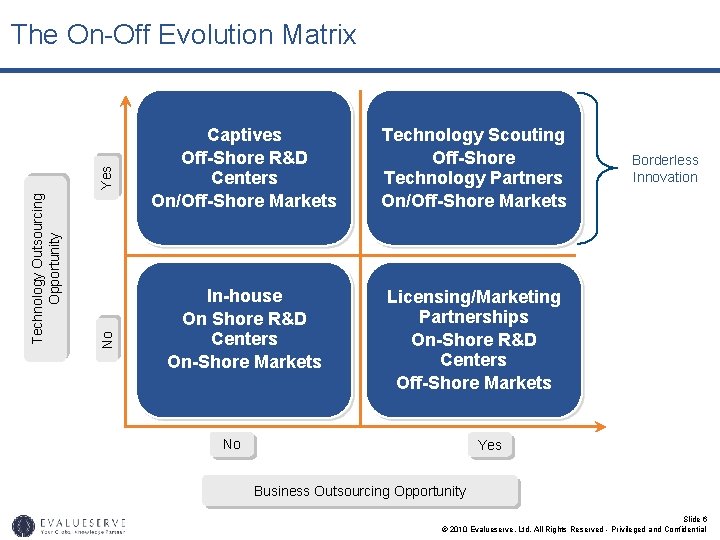
Yes No Technology Outsourcing Opportunity The On-Off Evolution Matrix Captives Off-Shore R&D Centers On/Off-Shore Markets Technology Scouting Off-Shore Technology Partners On/Off-Shore Markets In-house On Shore R&D Centers On-Shore Markets Licensing/Marketing Partnerships On-Shore R&D Centers Off-Shore Markets No Borderless Innovation Yes Business Outsourcing Opportunity Slide 6 © 2010 Evalueserve, Ltd. All Rights Reserved - Privileged and Confidential

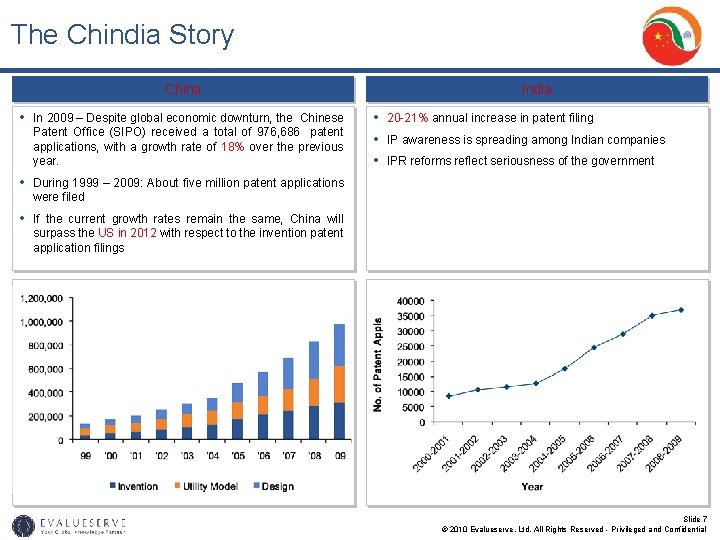
The Chindia Story China • In 2009 – Despite global economic downturn, the Chinese Patent Office (SIPO) received a total of 976, 686 patent applications, with a growth rate of 18% over the previous year. India • 20 -21% annual increase in patent filing • IP awareness is spreading among Indian companies • IPR reforms reflect seriousness of the government • During 1999 – 2009: About five million patent applications were filed • If the current growth rates remain the same, China will surpass the US in 2012 with respect to the invention patent application filings Slide 7 © 2010 Evalueserve, Ltd. All Rights Reserved - Privileged and Confidential

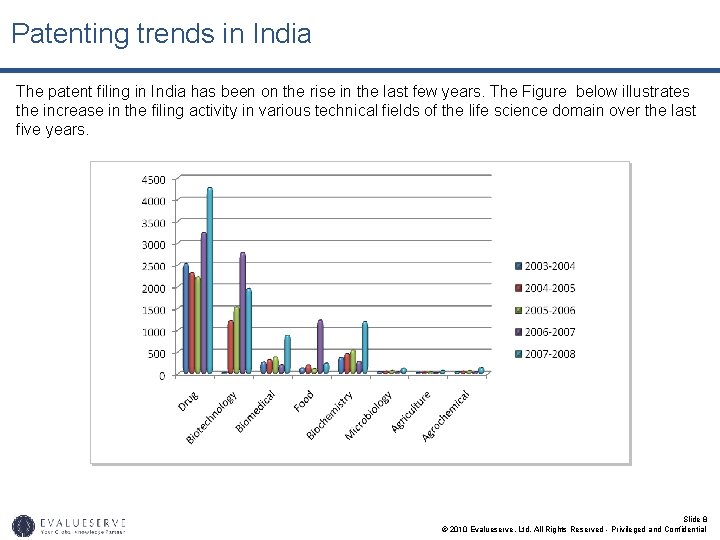
Patenting trends in India The patent filing in India has been on the rise in the last few years. The Figure below illustrates the increase in the filing activity in various technical fields of the life science domain over the last five years. Slide 8 © 2010 Evalueserve, Ltd. All Rights Reserved - Privileged and Confidential

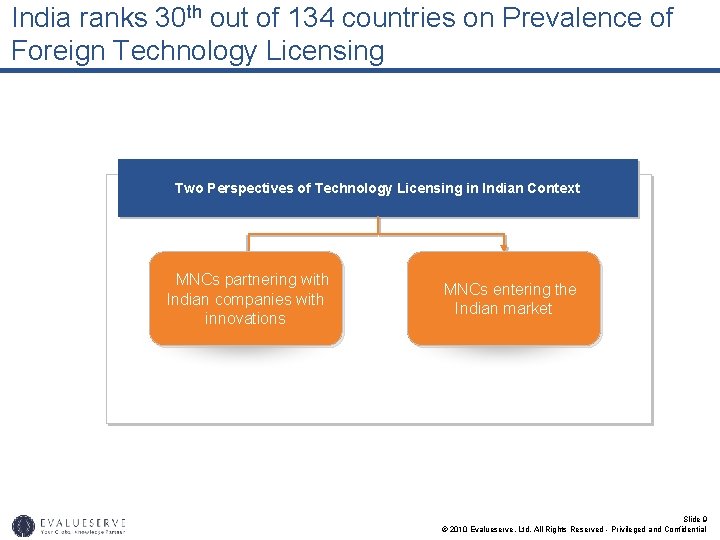
India ranks 30 th out of 134 countries on Prevalence of Foreign Technology Licensing Two Perspectives of Technology Licensing in Indian Context MNCs partnering with Indian companies with innovations MNCs entering the Indian market Slide 9 © 2010 Evalueserve, Ltd. All Rights Reserved - Privileged and Confidential

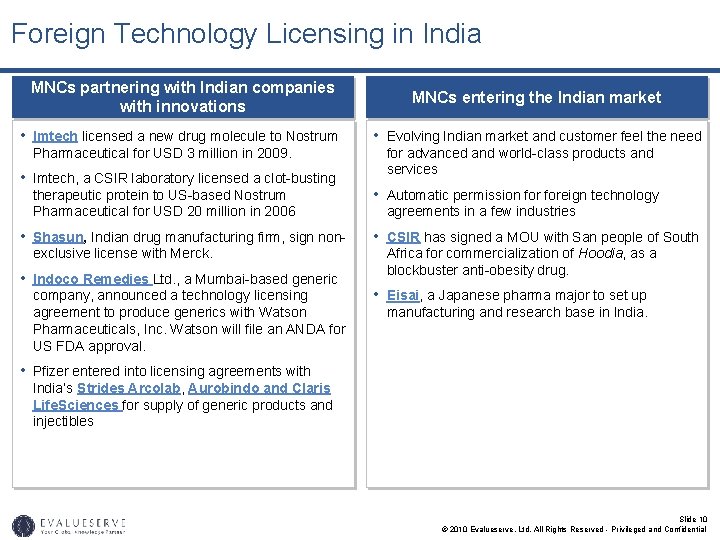
Foreign Technology Licensing in India MNCs partnering with Indian companies with innovations MNCs entering the Indian market • Imtech licensed a new drug molecule to Nostrum • Evolving Indian market and customer feel the need Pharmaceutical for USD 3 million in 2009. • Imtech, a CSIR laboratory licensed a clot-busting therapeutic protein to US-based Nostrum Pharmaceutical for USD 20 million in 2006 • Shasun, Indian drug manufacturing firm, sign nonexclusive license with Merck. • Indoco Remedies Ltd. , a Mumbai-based generic company, announced a technology licensing agreement to produce generics with Watson Pharmaceuticals, Inc. Watson will file an ANDA for US FDA approval. for advanced and world-class products and services • Automatic permission foreign technology agreements in a few industries • CSIR has signed a MOU with San people of South Africa for commercialization of Hoodia, as a blockbuster anti-obesity drug. • Eisai, a Japanese pharma major to set up manufacturing and research base in India. • Pfizer entered into licensing agreements with India’s Strides Arcolab, Aurobindo and Claris Life. Sciences for supply of generic products and injectibles Slide 10 © 2010 Evalueserve, Ltd. All Rights Reserved - Privileged and Confidential

Presentation Plan IP Valuation Overview IP Valuation Approaches Case Study Appendix Slide 11 © 2010 Evalueserve, Ltd. All Rights Reserved - Privileged and Confidential

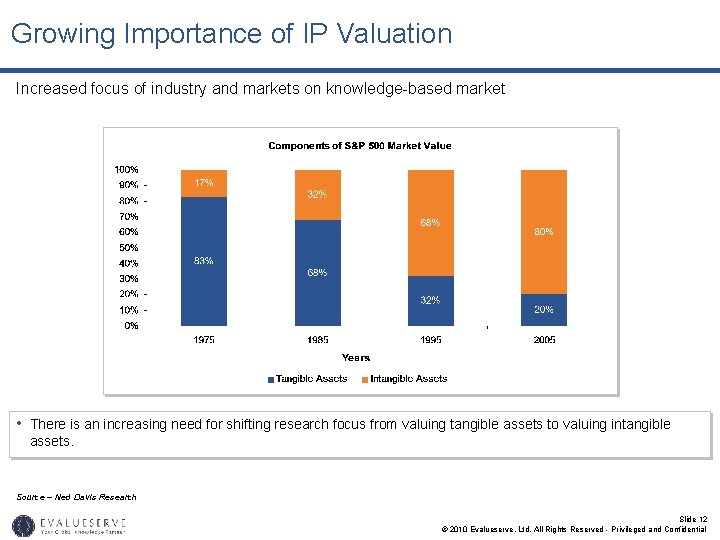
Growing Importance of IP Valuation Increased focus of industry and markets on knowledge-based market • There is an increasing need for shifting research focus from valuing tangible assets to valuing intangible assets. Source – Ned Davis Research Slide 12 © 2010 Evalueserve, Ltd. All Rights Reserved - Privileged and Confidential

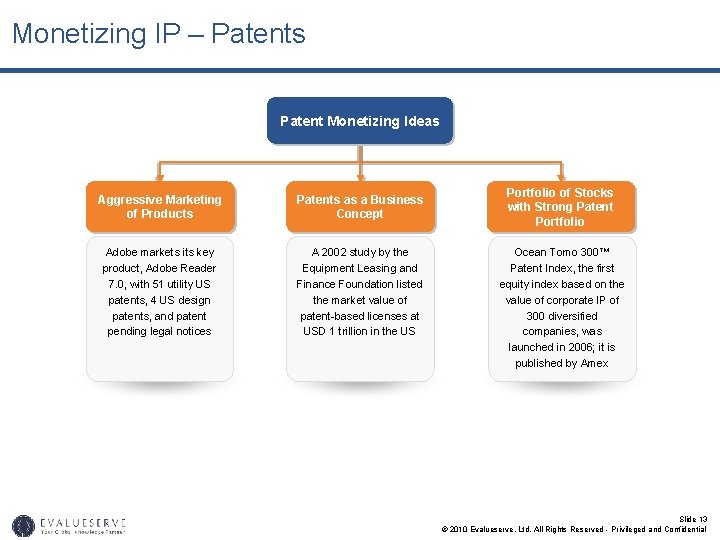
Monetizing IP – Patents Patent Monetizing Ideas Aggressive Marketing of Products Patents as a Business Concept Adobe markets its key product, Adobe Reader 7. 0, with 51 utility US patents, 4 US design patents, and patent pending legal notices A 2002 study by the Equipment Leasing and Finance Foundation listed the market value of patent-based licenses at USD 1 trillion in the US Portfolio of Stocks with Strong Patent Portfolio Ocean Tomo 300™ Patent Index, the first equity index based on the value of corporate IP of 300 diversified companies, was launched in 2006; it is published by Amex Slide 13 © 2010 Evalueserve, Ltd. All Rights Reserved - Privileged and Confidential

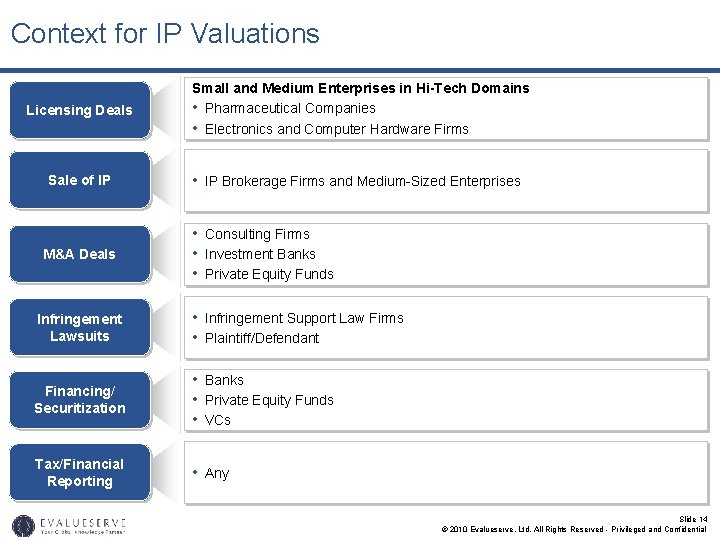
Context for IP Valuations Licensing Deals Sale of IP M&A Deals Small and Medium Enterprises in Hi-Tech Domains • Pharmaceutical Companies • Electronics and Computer Hardware Firms • IP Brokerage Firms and Medium-Sized Enterprises • Consulting Firms • Investment Banks • Private Equity Funds Infringement Lawsuits • Infringement Support Law Firms • Plaintiff/Defendant Financing/ Securitization • Banks • Private Equity Funds • VCs Tax/Financial Reporting • Any Slide 14 © 2010 Evalueserve, Ltd. All Rights Reserved - Privileged and Confidential

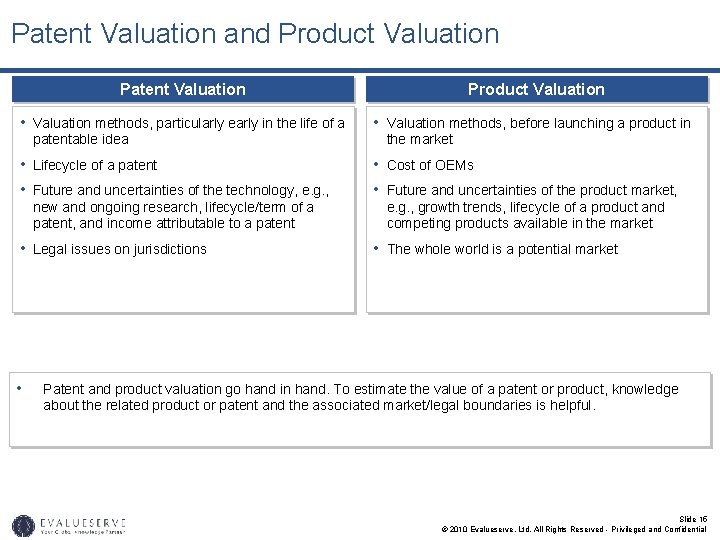
Patent Valuation and Product Valuation Patent Valuation Product Valuation • Valuation methods, particularly early in the life of a • Valuation methods, before launching a product in patentable idea the market • Lifecycle of a patent • Future and uncertainties of the technology, e. g. , • Cost of OEMs • Future and uncertainties of the product market, new and ongoing research, lifecycle/term of a patent, and income attributable to a patent e. g. , growth trends, lifecycle of a product and competing products available in the market • Legal issues on jurisdictions • • The whole world is a potential market Patent and product valuation go hand in hand. To estimate the value of a patent or product, knowledge about the related product or patent and the associated market/legal boundaries is helpful. Slide 15 © 2010 Evalueserve, Ltd. All Rights Reserved - Privileged and Confidential

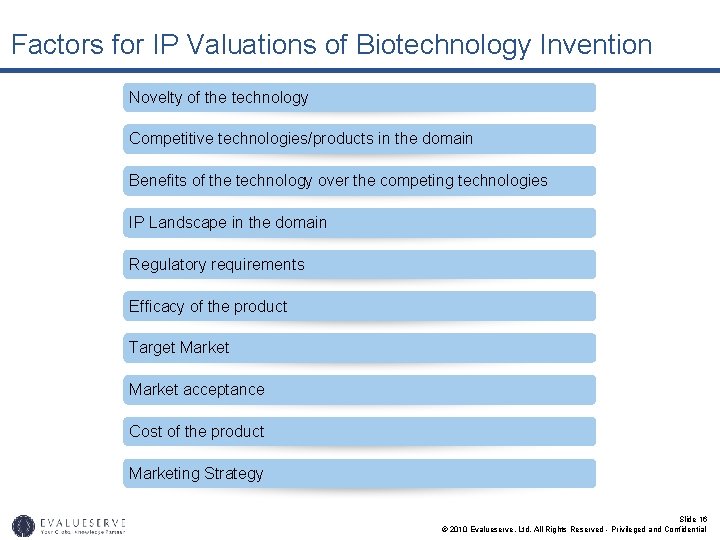
Factors for IP Valuations of Biotechnology Invention Novelty of the technology Competitive technologies/products in the domain Benefits of the technology over the competing technologies IP Landscape in the domain Regulatory requirements Efficacy of the product Target Market acceptance Cost of the product Marketing Strategy Slide 16 © 2010 Evalueserve, Ltd. All Rights Reserved - Privileged and Confidential

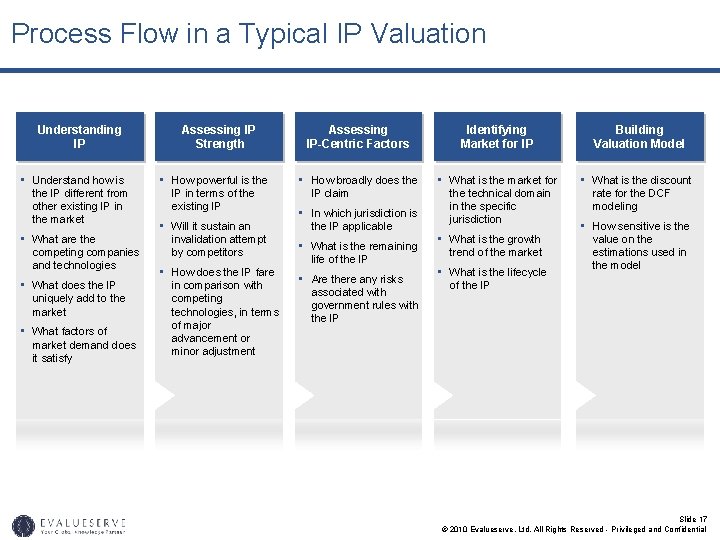
Process Flow in a Typical IP Valuation Understanding IP • Understand how is the IP different from other existing IP in the market • What are the competing companies and technologies • What does the IP uniquely add to the market • What factors of market demand does it satisfy Assessing IP Strength • How powerful is the IP in terms of the existing IP • Will it sustain an invalidation attempt by competitors • How does the IP fare in comparison with competing technologies, in terms of major advancement or minor adjustment Assessing IP-Centric Factors Identifying Market for IP Building Valuation Model • How broadly does the • What is the market for • What is the discount IP claim • In which jurisdiction is the IP applicable • What is the remaining life of the IP • Are there any risks associated with government rules with the IP the technical domain in the specific jurisdiction • What is the growth trend of the market • What is the lifecycle rate for the DCF modeling • How sensitive is the value on the estimations used in the model of the IP Slide 17 © 2010 Evalueserve, Ltd. All Rights Reserved - Privileged and Confidential

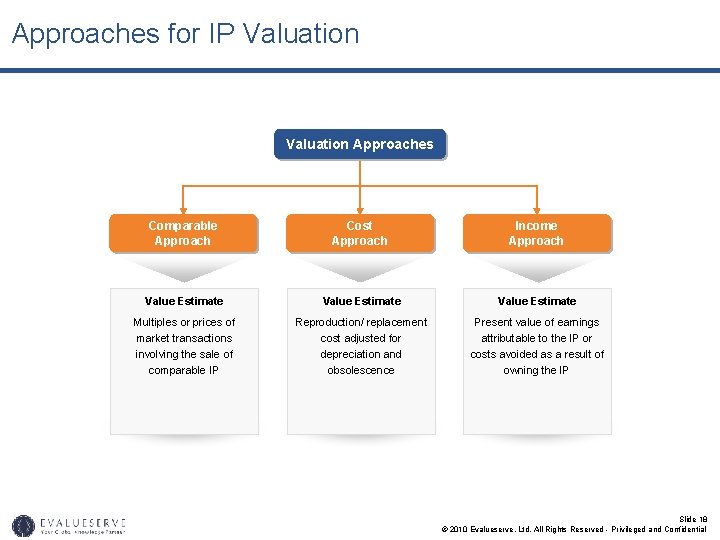
Approaches for IP Valuation Approaches Comparable Approach Cost Approach Income Approach Value Estimate Multiples or prices of market transactions involving the sale of comparable IP Reproduction/ replacement cost adjusted for depreciation and obsolescence Present value of earnings attributable to the IP or costs avoided as a result of owning the IP Slide 18 © 2010 Evalueserve, Ltd. All Rights Reserved - Privileged and Confidential

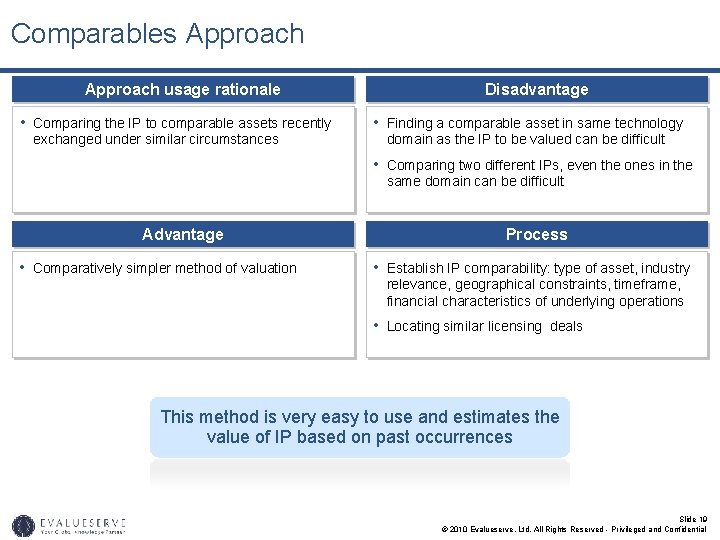
Comparables Approach usage rationale • Comparing the IP to comparable assets recently exchanged under similar circumstances Disadvantage • Finding a comparable asset in same technology domain as the IP to be valued can be difficult • Comparing two different IPs, even the ones in the same domain can be difficult Advantage • Comparatively simpler method of valuation Process • Establish IP comparability: type of asset, industry relevance, geographical constraints, timeframe, financial characteristics of underlying operations • Locating similar licensing deals This method is very easy to use and estimates the value of IP based on past occurrences Slide 19 © 2010 Evalueserve, Ltd. All Rights Reserved - Privileged and Confidential

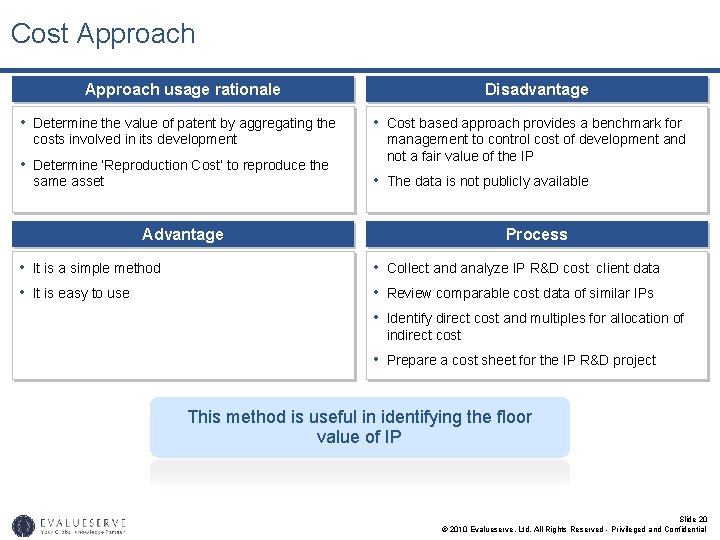
Cost Approach usage rationale • Determine the value of patent by aggregating the costs involved in its development • Determine ‘Reproduction Cost’ to reproduce the same asset Disadvantage • Cost based approach provides a benchmark for management to control cost of development and not a fair value of the IP • The data is not publicly available Advantage • It is a simple method • It is easy to use Process • Collect and analyze IP R&D cost client data • Review comparable cost data of similar IPs • Identify direct cost and multiples for allocation of indirect cost • Prepare a cost sheet for the IP R&D project This method is useful in identifying the floor value of IP Slide 20 © 2010 Evalueserve, Ltd. All Rights Reserved - Privileged and Confidential

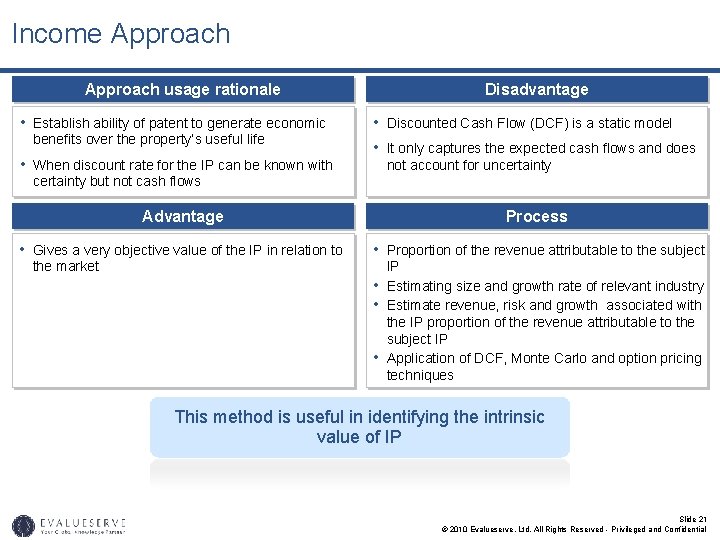
Income Approach usage rationale • Establish ability of patent to generate economic benefits over the property’s useful life • When discount rate for the IP can be known with Disadvantage • Discounted Cash Flow (DCF) is a static model • It only captures the expected cash flows and does not account for uncertainty but not cash flows Advantage Process • Gives a very objective value of the IP in relation to • Proportion of the revenue attributable to the subject the market • • • IP Estimating size and growth rate of relevant industry Estimate revenue, risk and growth associated with the IP proportion of the revenue attributable to the subject IP Application of DCF, Monte Carlo and option pricing techniques This method is useful in identifying the intrinsic value of IP Slide 21 © 2010 Evalueserve, Ltd. All Rights Reserved - Privileged and Confidential

Presentation Plan IP Valuation Overview IP Valuation Approaches Case Study Appendix Slide 22 © 2010 Evalueserve, Ltd. All Rights Reserved - Privileged and Confidential

Case Study*: How do we actually value a patent? What is patented? • A biosimilar drug molecule for treatment of autoimmune diseases. Objective and Need • You need to present the patented idea to a pharmaceutical company to sell the patent. • They are already convinced that there is a market that can be tapped. • You need to identify the target market and what value should you go to the negotiation table with. What methodology will you follow to identify the price of the patent? • Assess the strength of IP and the competing IP in the space • Identify market targeted by the IP based on IP centric parameters • Identify licensing amount *: Indicative numbers have been used in this case study Slide 23 © 2010 Evalueserve, Ltd. All Rights Reserved - Privileged and Confidential

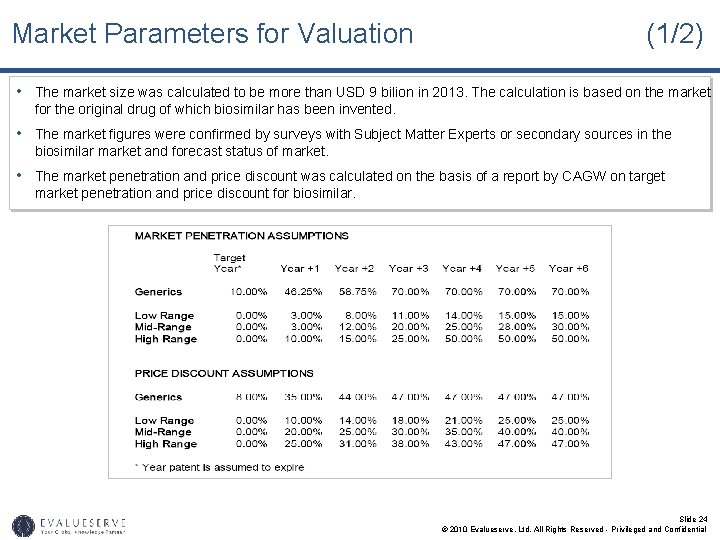
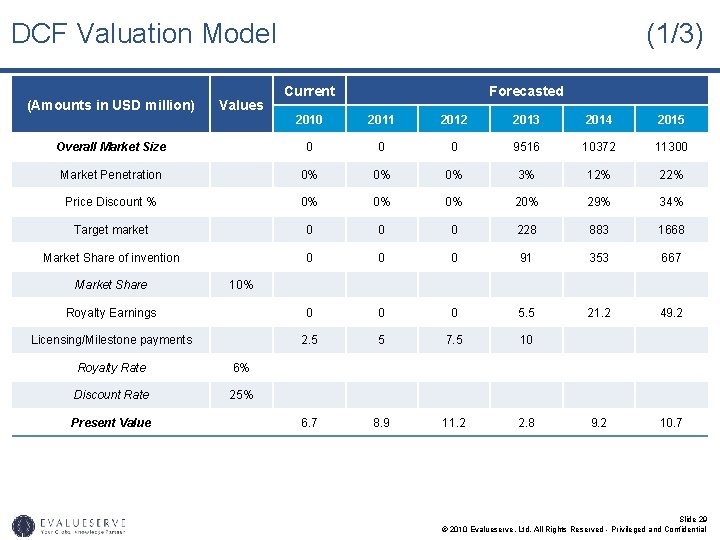
Market Parameters for Valuation (1/2) • The market size was calculated to be more than USD 9 bilion in 2013. The calculation is based on the market for the original drug of which biosimilar has been invented. • The market figures were confirmed by surveys with Subject Matter Experts or secondary sources in the biosimilar market and forecast status of market. • The market penetration and price discount was calculated on the basis of a report by CAGW on target market penetration and price discount for biosimilar. Slide 24 © 2010 Evalueserve, Ltd. All Rights Reserved - Privileged and Confidential

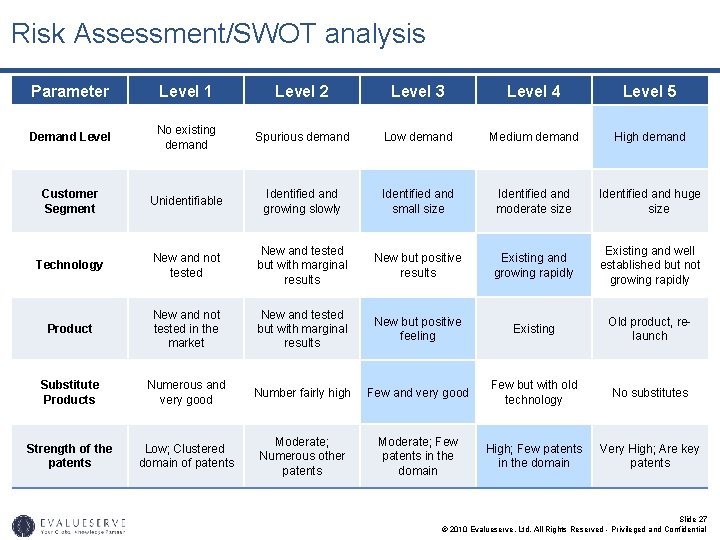
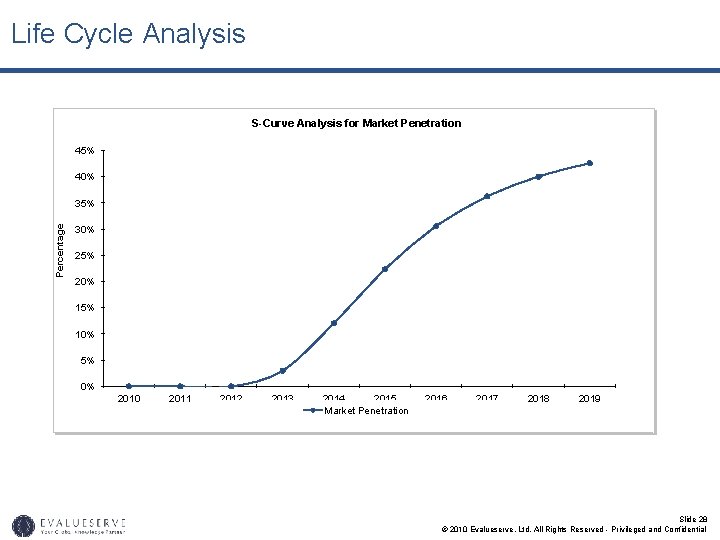
Market Parameters for Valuation (2/2) • Based on IP analysis, and competitive intelligence you can determine if your new drug is a high-range, medium-range or low-range drug. • Assuming it to be a medium-range biosimilar, you can expect a market penetration of 3% in the year of launch and 40% after 6 years. • Again for a medium-range biosimilar the price of the product will also be reduced based on price discount • The market share for intermediate years was calculated on the basis of S-curve. • The cost of conducting clinical trials for FDA approval will require approximately USD 25 million over the next 4 years. • The average royalty rates on sales in the pharmaceutical sector have been found to be 6%. • The discount rate has been assumed based on the industry standard for pharmaceutical sector. This has been confirmed by the risk assessment matrix or the SWOT analysis of the technology. Slide 25 © 2010 Evalueserve, Ltd. All Rights Reserved - Privileged and Confidential

IP Parameters for Valuation • IP Landscape analysis was conducted to identify relevant IP in the domain. Intensive IP analysis, such as broadness of claims, details of office actions can help in identifying the share of the market that can be attributed to current technology. • Active companies in the technology domain were identified as they can influence the market captured by the current biosimilar. • Both these parameters have been factored in the risk assessment matrix while calculating the discount rate that can be offered. Slide 26 © 2010 Evalueserve, Ltd. All Rights Reserved - Privileged and Confidential

Risk Assessment/SWOT analysis Parameter Level 1 Level 2 Level 3 Level 4 Level 5 Demand Level No existing demand Spurious demand Low demand Medium demand High demand Customer Segment Unidentifiable Identified and growing slowly Identified and small size Identified and moderate size Identified and huge size Technology New and not tested New and tested but with marginal results New but positive results Existing and growing rapidly Existing and well established but not growing rapidly Product New and not tested in the market New and tested but with marginal results New but positive feeling Existing Old product, relaunch Substitute Products Numerous and very good Number fairly high Few and very good Few but with old technology No substitutes Strength of the patents Low; Clustered domain of patents Moderate; Numerous other patents Moderate; Few patents in the domain High; Few patents in the domain Very High; Are key patents Slide 27 © 2010 Evalueserve, Ltd. All Rights Reserved - Privileged and Confidential

Life Cycle Analysis S-Curve Analysis for Market Penetration 45% 40% Percentage 35% 30% 25% 20% 15% 10% 5% 0% 2010 2011 2012 2013 2014 2015 Market Penetration 2016 2017 2018 2019 Slide 28 © 2010 Evalueserve, Ltd. All Rights Reserved - Privileged and Confidential

DCF Valuation Model (Amounts in USD million) Values Overall Market Size Market Penetration Current Forecasted 2010 2011 2012 2013 2014 2015 0 0 0 9516 10372 11300 0% 0% 0% 3% 12% 22% 0% 0% 0% 29% 34% 0 0 0 228 883 1668 0 0 0 91 353 667 Price Discount % Target market (1/3) Market Share of invention Market Share 10% Royalty Earnings 0 0 0 5. 5 21. 2 49. 2 2. 5 5 7. 5 10 Licensing/Milestone payments Royalty Rate 6% Discount Rate 25% Present Value 6. 7 8. 9 11. 2 2. 8 9. 2 10. 7 Slide 29 © 2010 Evalueserve, Ltd. All Rights Reserved - Privileged and Confidential

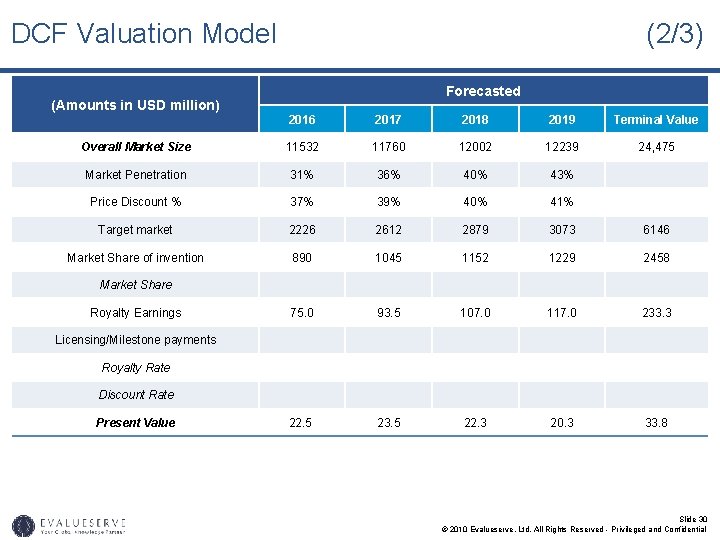
DCF Valuation Model (Amounts in USD million) (2/3) Forecasted 2016 2017 2018 2019 Terminal Value Overall Market Size 11532 11760 12002 12239 24, 475 Market Penetration 31% 36% 40% 43% Price Discount % 37% 39% 40% 41% Target market 2226 2612 2879 3073 6146 Market Share of invention 890 1045 1152 1229 2458 Market Share Royalty Earnings 75. 0 93. 5 107. 0 117. 0 233. 3 Discount Rate Present Value 22. 5 23. 5 22. 3 20. 3 33. 8 Licensing/Milestone payments Royalty Rate Slide 30 © 2010 Evalueserve, Ltd. All Rights Reserved - Privileged and Confidential

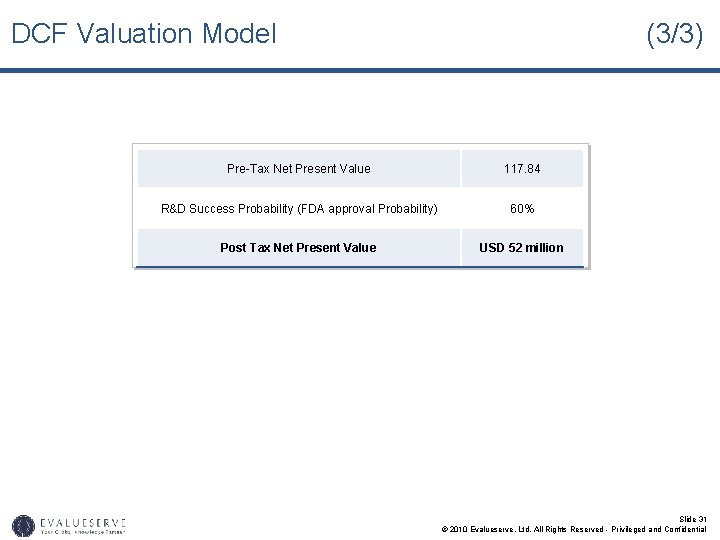
DCF Valuation Model (3/3) Pre-Tax Net Present Value 117. 84 R&D Success Probability (FDA approval Probability) 60% Post Tax Net Present Value USD 52 million Slide 31 © 2010 Evalueserve, Ltd. All Rights Reserved - Privileged and Confidential

Presentation Plan IP Valuation Overview IP Valuation Approaches Case Study Appendix Slide 32 © 2010 Evalueserve, Ltd. All Rights Reserved - Privileged and Confidential

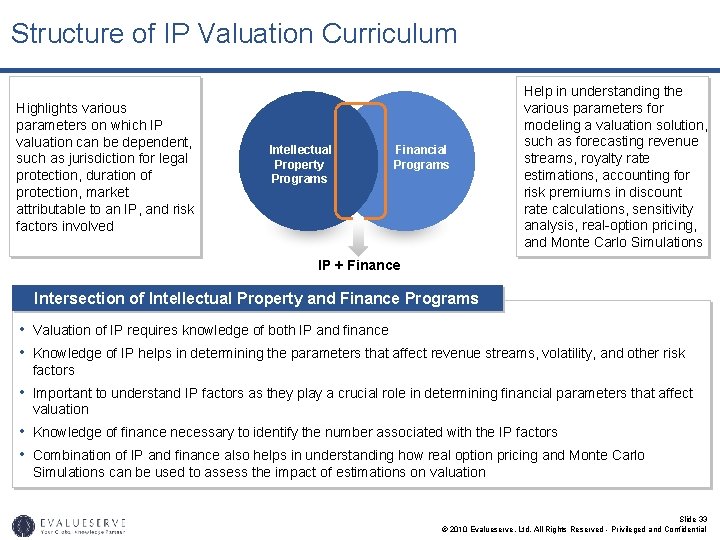
Structure of IP Valuation Curriculum Highlights various parameters on which IP valuation can be dependent, such as jurisdiction for legal protection, duration of protection, market attributable to an IP, and risk factors involved Intellectual Property Programs Financial Programs Help in understanding the various parameters for modeling a valuation solution, such as forecasting revenue streams, royalty rate estimations, accounting for risk premiums in discount rate calculations, sensitivity analysis, real-option pricing, and Monte Carlo Simulations IP + Finance Intersection of Intellectual Property and Finance Programs • Valuation of IP requires knowledge of both IP and finance • Knowledge of IP helps in determining the parameters that affect revenue streams, volatility, and other risk factors • Important to understand IP factors as they play a crucial role in determining financial parameters that affect valuation • Knowledge of finance necessary to identify the number associated with the IP factors • Combination of IP and finance also helps in understanding how real option pricing and Monte Carlo Simulations can be used to assess the impact of estimations on valuation Slide 33 © 2010 Evalueserve, Ltd. All Rights Reserved - Privileged and Confidential

Existing Curriculums The National Law University, Jodhpur, is the first university in India to offer Masters Degree, and Honors and Distance Education Programs in IPR Honors program has eight papers, one of which is IP Valuation. The PSB Academy, an institute in Singapore, offers a one-day lecture-cum-enhanced learning through case studies training program–Intellectual Property Management for Enterprise Managers. Their training on IP Valuation comprises purpose of intangible asset valuation, commonly-used IP valuation approaches, and IP asset valuation criteria. Bournemouth University, Center for Intellectual Property Policy & Management, specializes in copyright, industrial design, patents, trade marks, law & policy, IP exploitation, and legal education. It offers Customized IPR Short Courses—a half-day, a one-day and a two-day course, including IP licensing and IP valuation. Telestrat Education is a worldwide leader in continuing education seminars. It provides a half-day course on IP Valuation, which includes valuation basics, valuation methods, discount rate, and cost of capital knowledge. Slide 34 © 2010 Evalueserve, Ltd. All Rights Reserved - Privileged and Confidential

Research Topics and References • Estimating growth rate of revenue streams from an IP by using SWOT analysis based on IPspecific risk parameters • Accounting for IP parameters, such as FDA approvals in the case of a biotech IP, as risk Research Topics premiums • Estimation of royalty rates for IP in various technical domains • Real-options and binomial approach—when treating patents as an asset with an option of creating products based on the patents now or later References • Strategies for Investing in Intellectual Property by David S. Ruder • Essentials of Licensing Intellectual Property by Alexander I. Poltorak, Paul J. Lerner • Intellectual Property Assets in Mergers and Acquisitions by Melvin Seminsky Slide 35 © 2010 Evalueserve, Ltd. All Rights Reserved - Privileged and Confidential

Thank you © 2010 Evalueserve, Ltd. All Rights Reserved - Privileged and Confidential