Ions In general atoms are electrically neutral They







- Slides: 28

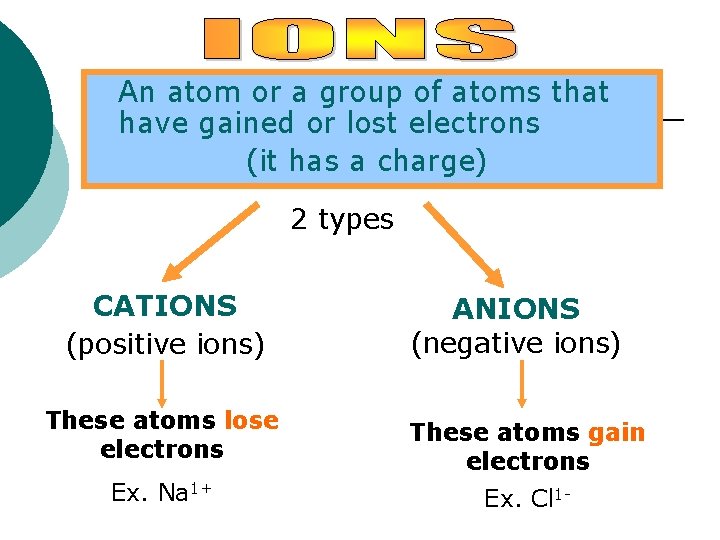
Ions In general, atoms are electrically neutral ¡ They have the same number of protons (+) as electrons (-) ¡ However, because atoms can gain or lose electrons, they can become charged. ¡

An atom or a group of atoms that have gained or lost electrons (it has a charge) 2 types CATIONS (positive ions) ANIONS (negative ions) These atoms lose electrons These atoms gain electrons Ex. Na 1+ Ex. Cl 1 -

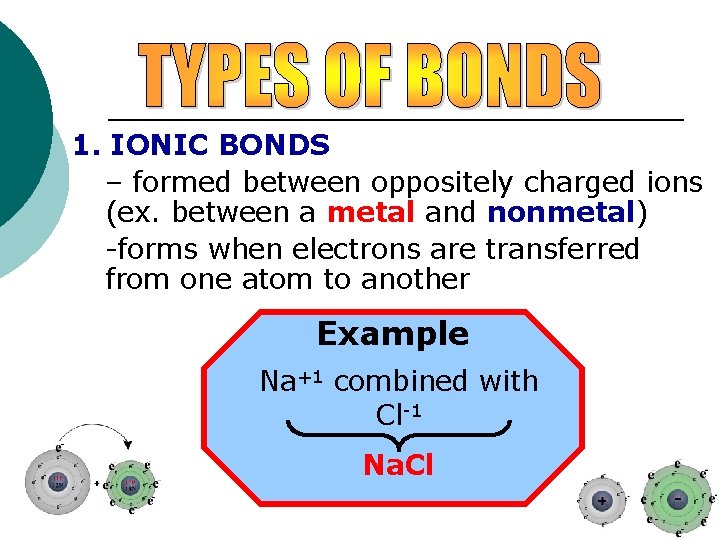
1. IONIC BONDS – formed between oppositely charged ions (ex. between a metal and nonmetal) -forms when electrons are transferred from one atom to another Example Na+1 combined with Cl-1 Na. Cl

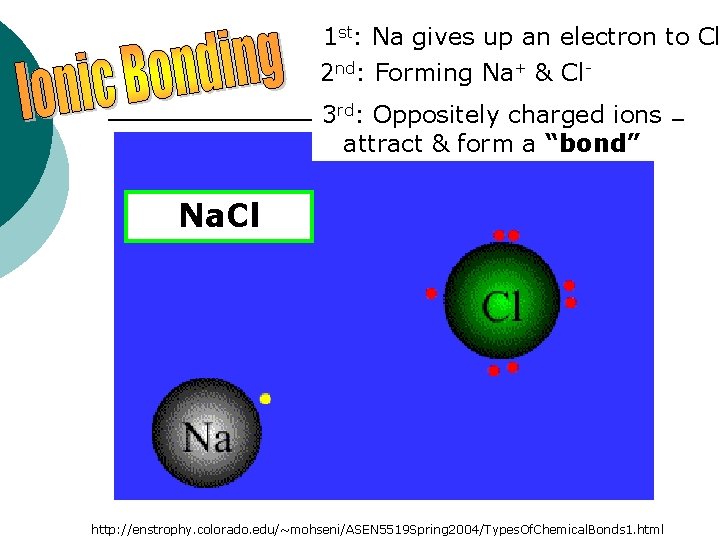
1 st: Na gives up an electron to Cl 2 nd: Forming Na+ & Cl 3 rd: Oppositely charged ions attract & form a “bond” Na. Cl http: //enstrophy. colorado. edu/~mohseni/ASEN 5519 Spring 2004/Types. Of. Chemical. Bonds 1. html

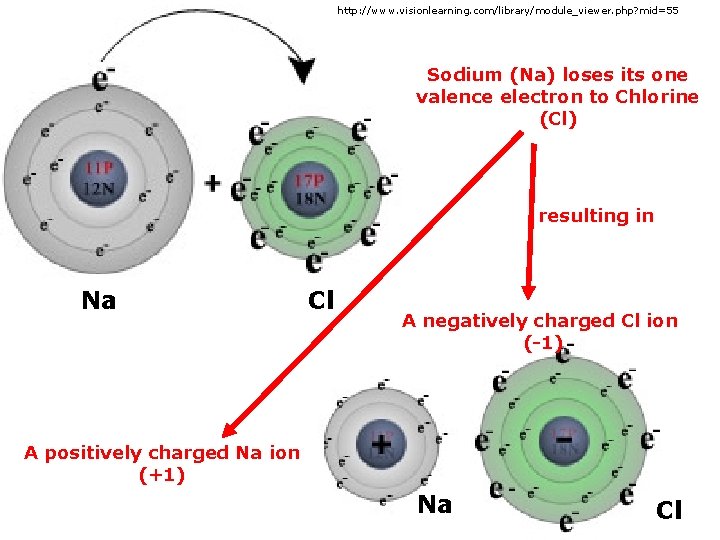
http: //www. visionlearning. com/library/module_viewer. php? mid=55 Sodium (Na) loses its one valence electron to Chlorine (Cl) resulting in Na Cl A negatively charged Cl ion (-1) A positively charged Na ion (+1) Na Cl

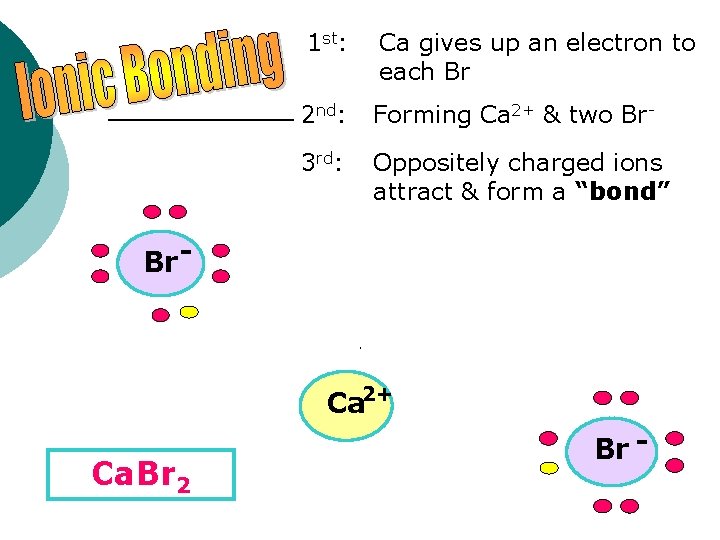
1 st: Ca gives up an electron to each Br 2 nd: Forming Ca 2+ & two Br- 3 rd: Oppositely charged ions attract & form a “bond” Br - Ca 2+ Ca. Br 2 Br -

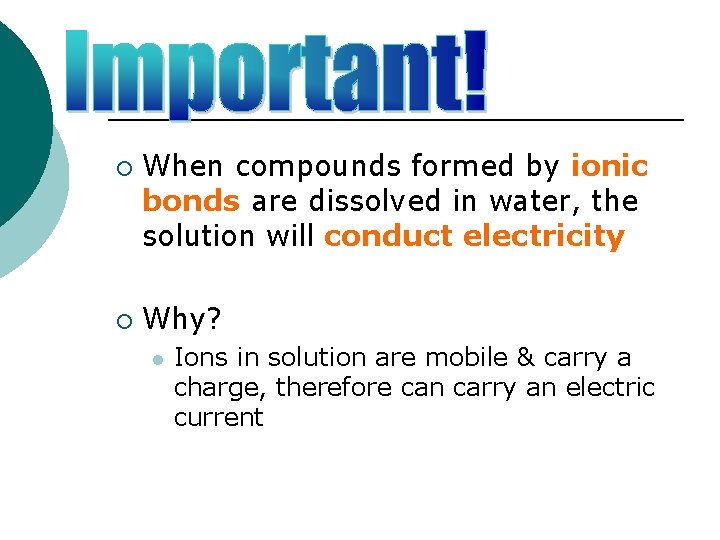
¡ ¡ When compounds formed by ionic bonds are dissolved in water, the solution will conduct electricity Why? l Ions in solution are mobile & carry a charge, therefore can carry an electric current

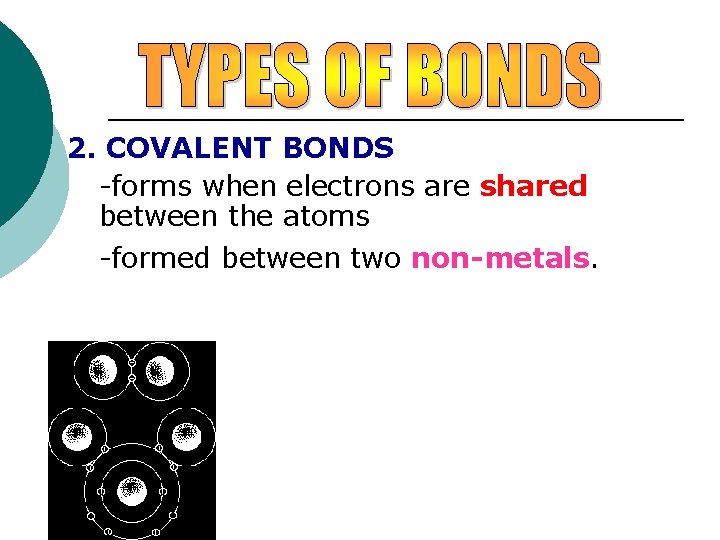
2. COVALENT BONDS -forms when electrons are shared between the atoms -formed between two non-metals. Examples Cl 2 H 2 CO

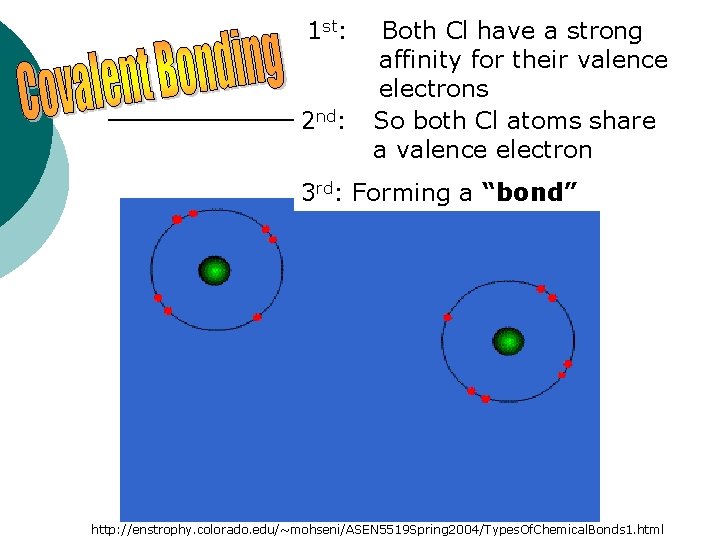
1 st: 2 nd: Both Cl have a strong affinity for their valence electrons So both Cl atoms share a valence electron 3 rd: Forming a “bond” http: //enstrophy. colorado. edu/~mohseni/ASEN 5519 Spring 2004/Types. Of. Chemical. Bonds 1. html

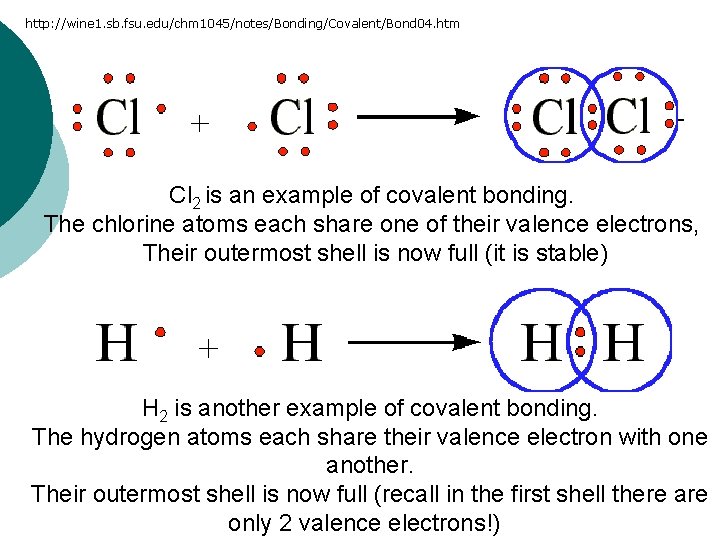
http: //wine 1. sb. fsu. edu/chm 1045/notes/Bonding/Covalent/Bond 04. htm Cl 2 is an example of covalent bonding. The chlorine atoms each share one of their valence electrons, Their outermost shell is now full (it is stable) H 2 is another example of covalent bonding. The hydrogen atoms each share their valence electron with one another. Their outermost shell is now full (recall in the first shell there are only 2 valence electrons!)

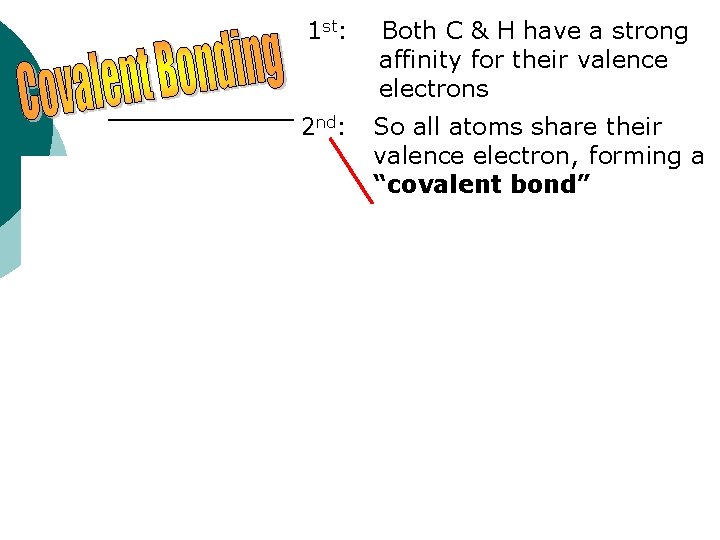
1 st: Both C & H have a strong affinity for their valence electrons 2 nd: So all atoms share their valence electron, forming a “covalent bond”

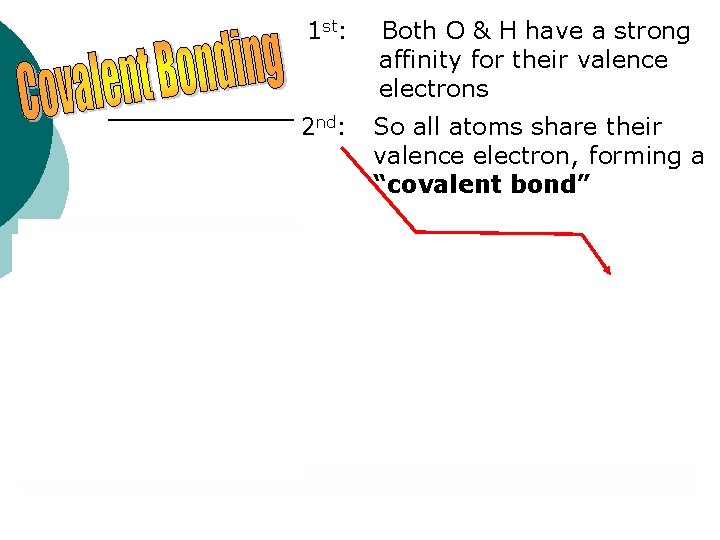
1 st: Both O & H have a strong affinity for their valence electrons 2 nd: So all atoms share their valence electron, forming a “covalent bond”

¡ ¡ When compounds formed by covalent bonds are dissolved in water, the solution will NOT conduct electricity Why? l There are no ions or free charges present, so no electricity can be conducted

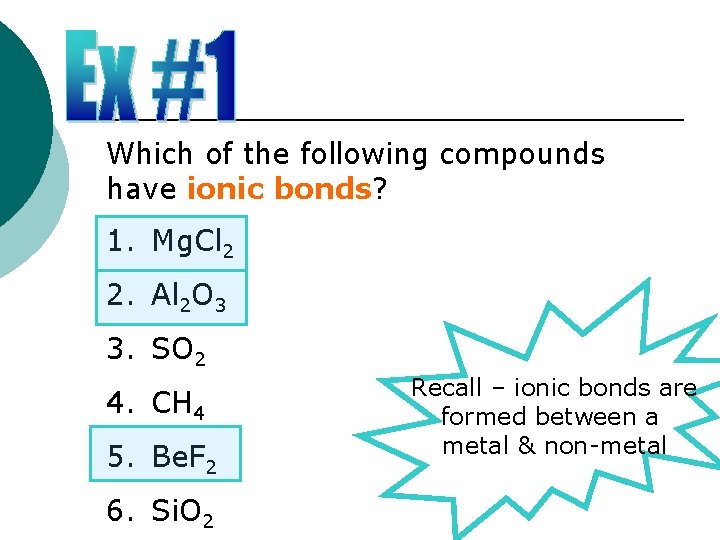
Which of the following compounds have ionic bonds? 1. Mg. Cl 2 2. Al 2 O 3 3. SO 2 4. CH 4 5. Be. F 2 6. Si. O 2 Recall – ionic bonds are formed between a metal & non-metal

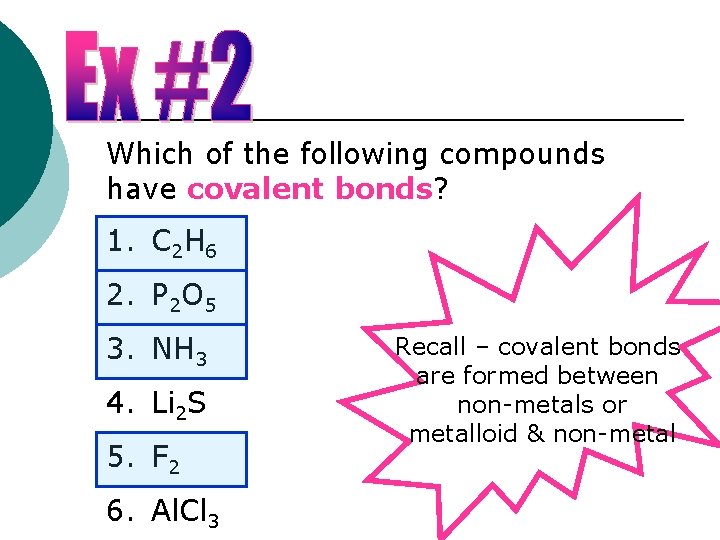
Which of the following compounds have covalent bonds? 1. C 2 H 6 2. P 2 O 5 3. NH 3 4. Li 2 S 5. F 2 6. Al. Cl 3 Recall – covalent bonds are formed between non-metals or metalloid & non-metal

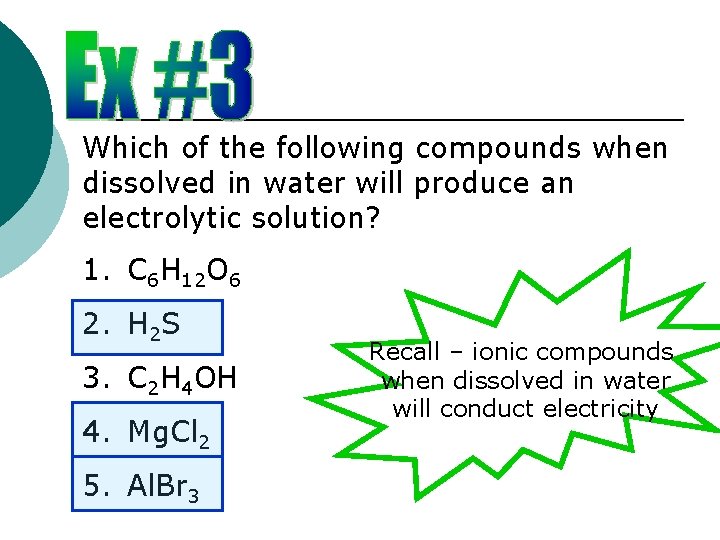
Which of the following compounds when dissolved in water will produce an electrolytic solution? 1. C 6 H 12 O 6 2. H 2 S 3. C 2 H 4 OH 4. Mg. Cl 2 5. Al. Br 3 Recall – ionic compounds when dissolved in water will conduct electricity

Which of the following compounds when dissolved in water will NOT form ions? 1. Fe 2 O 3 2. H 2 3. Ca(OH)2 4. Mg. S 5. NO 2 Recall – only ionic compounds when dissolved in water will form ions

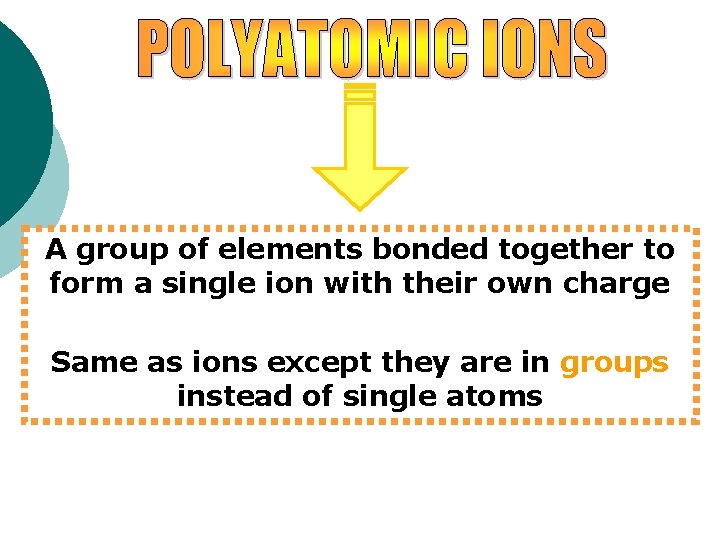
A group of elements bonded together to form a single ion with their own charge Same as ions except they are in groups instead of single atoms

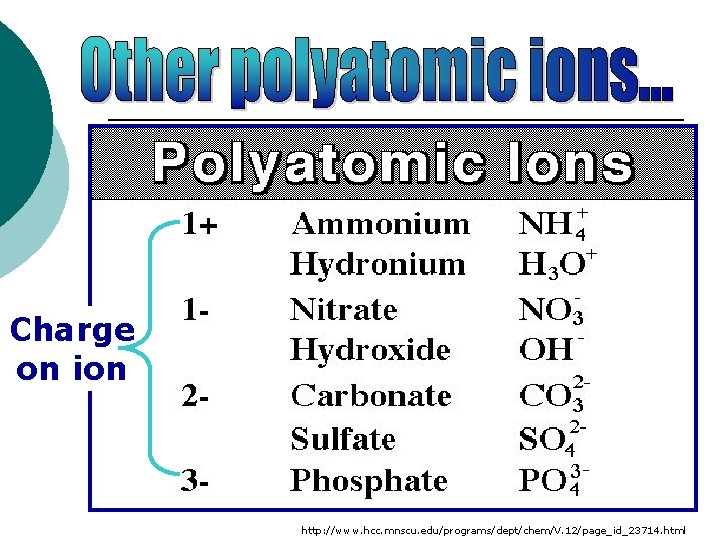
_ OH (hydroxide ion) • It has a charge of 1 • Consists of an oxygen atom and a hydrogen atom • Treated like one single ion even though it is made up of 2 atoms

SO 4 2 - (sulfate ion) • It has a charge of 2 • Consists of a sulfur atom and four oxygen atoms

Charge on ion http: //www. hcc. mnscu. edu/programs/dept/chem/V. 12/page_id_23714. html

¡ You must memorize the following names and charges for these polyatomic ions: OH-1 Hydroxide NH 4+1 Ammonium SO 4 -2 Sulfate CO 3 -2 Carbonate PO 4 -3 Phosphate NO 3 -1 Nitrate

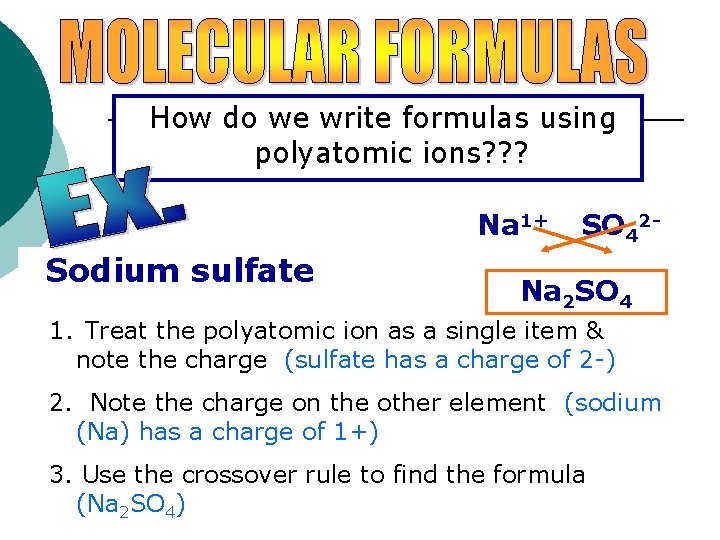
How do we write formulas using polyatomic ions? ? ? Na 1+ Sodium sulfate SO 42 - Na 2 SO 4 1. Treat the polyatomic ion as a single item & note the charge (sulfate has a charge of 2 -) 2. Note the charge on the other element (sodium (Na) has a charge of 1+) 3. Use the crossover rule to find the formula (Na 2 SO 4)

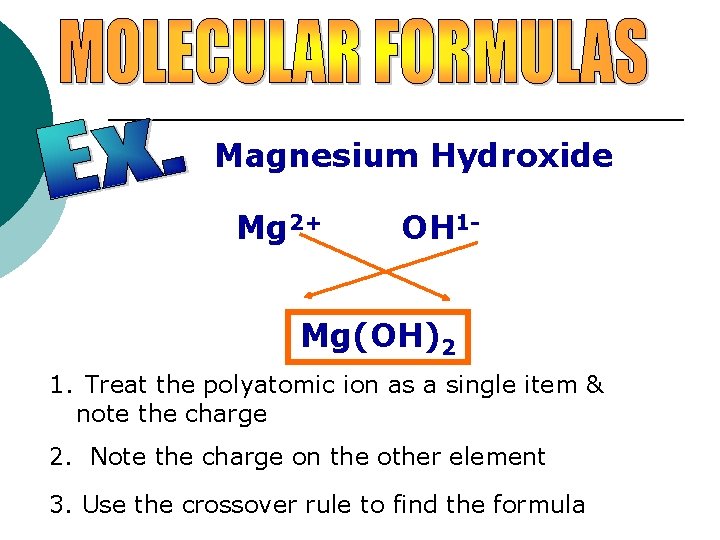
Magnesium Hydroxide Mg 2+ OH 1 - Mg(OH)2 1. Treat the polyatomic ion as a single item & note the charge 2. Note the charge on the other element 3. Use the crossover rule to find the formula

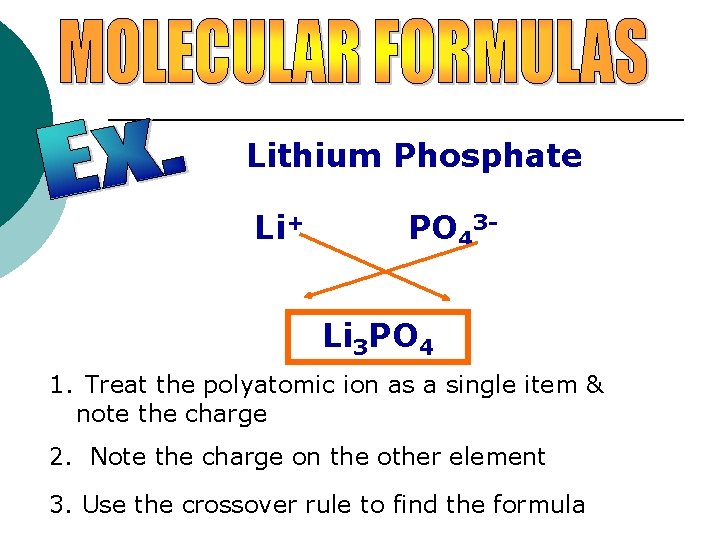
Lithium Phosphate Li+ PO 43 Li 3 PO 4 1. Treat the polyatomic ion as a single item & note the charge 2. Note the charge on the other element 3. Use the crossover rule to find the formula

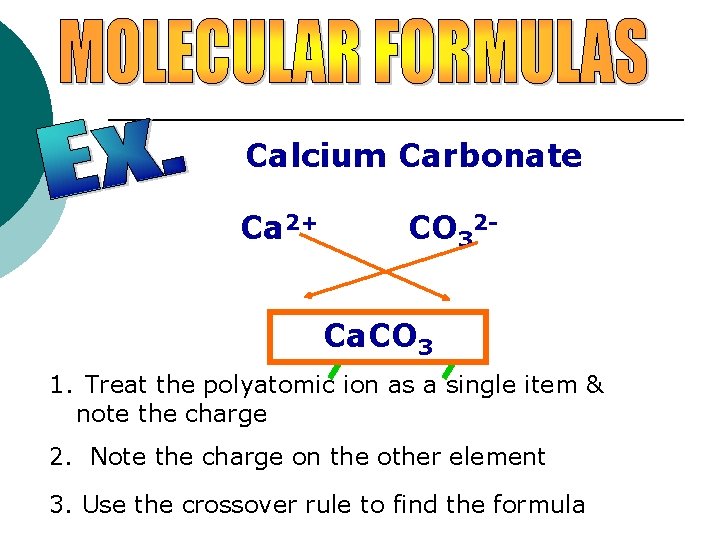
Calcium Carbonate Ca 2+ CO 32 - Ca. CO 2(CO 3 3 )2 1. Treat the polyatomic ion as a single item & note the charge 2. Note the charge on the other element 3. Use the crossover rule to find the formula

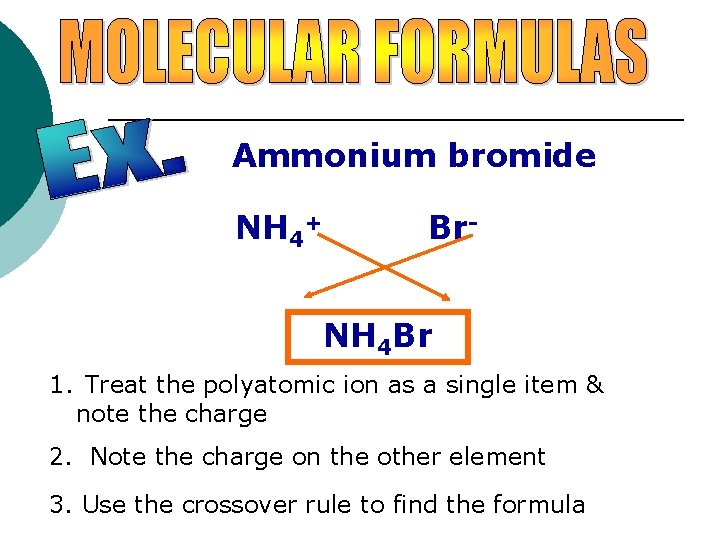
Ammonium bromide NH 4+ Br. NH 4 Br 1. Treat the polyatomic ion as a single item & note the charge 2. Note the charge on the other element 3. Use the crossover rule to find the formula

Worksheet # 1 ¡ Study guide, Module 3, page 8 and 9 ¡