Ions in Aqueous Solution PbNO 32s H 2
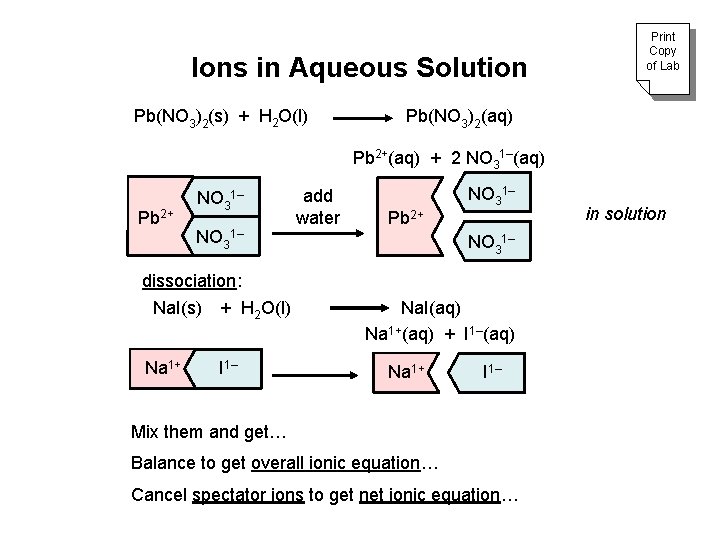
Ions in Aqueous Solution Pb(NO 3)2(s) + H 2 O(l) Print Copy of Lab Pb(NO 3)2(aq) Pb 2+(aq) + 2 NO 31–(aq) Pb 2+ NO 31– dissociation: Na. I(s) + H 2 O(l) Na 1+ I 1– add water Pb 2+ NO 31– Na. I(aq) Na 1+(aq) + I 1–(aq) Na 1+ I 1– Mix them and get… Balance to get overall ionic equation… Cancel spectator ions to get net ionic equation… in solution
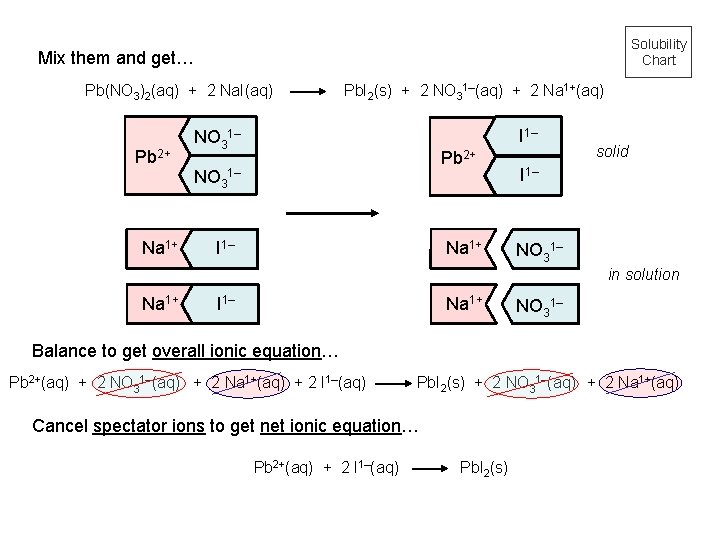
Solubility Chart Mix them and get… Pb(NO 3)2(aq) + 2 Na. I(aq) Pb 2+ Na 1+ Pb. I 2(s) + 2 NO 31–(aq) + 2 Na 1+(aq) I 1– NO 31– Pb 2+ NO 31– I 1– Na 1+ solid I 1– NO 31– in solution Na 1+ I 1– Na 1+ NO 31– Balance to get overall ionic equation… Pb 2+(aq) + 2 NO 31–(aq) + 2 Na 1+(aq) + 2 I 1–(aq) Pb. I 2(s) + 2 NO 31–(aq) + 2 Na 1+(aq) Cancel spectator ions to get net ionic equation… Pb 2+(aq) + 2 I 1–(aq) Pb. I 2(s)
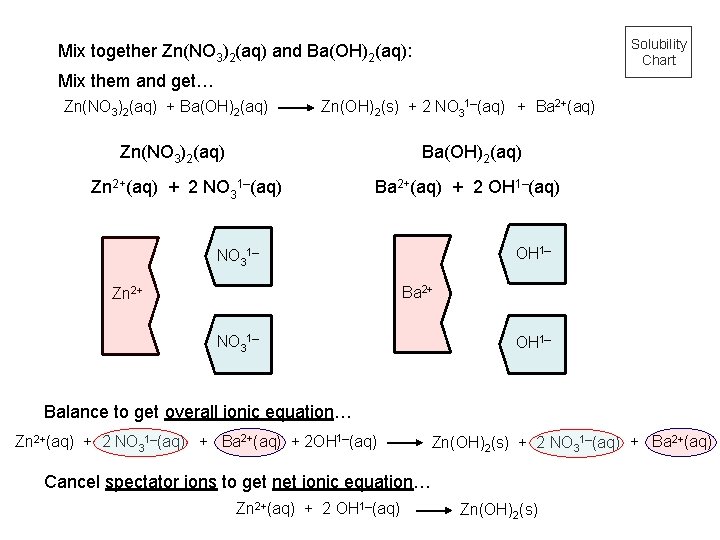
Solubility Chart Mix together Zn(NO 3)2(aq) and Ba(OH)2(aq): Mix them and get… Zn(NO 3)2(aq) + Ba(OH)2(aq) Zn(OH)2(s) + 2 NO 31–(aq) + Ba 2+(aq) Zn(NO 3)2(aq) Ba(OH)2(aq) Zn 2+(aq) + 2 NO 31–(aq) Ba 2+(aq) + 2 OH 1–(aq) OH 1– NO 31– Ba 2+ Zn 2+ NO 31– OH 1– Balance to get overall ionic equation… Zn 2+(aq) + 2 NO 31–(aq) + Ba 2+(aq) + 2 OH 1–(aq) Zn(OH)2(s) + 2 NO 31–(aq) + Ba 2+(aq) Cancel spectator ions to get net ionic equation… Zn 2+(aq) + 2 OH 1–(aq) Zn(OH)2(s)
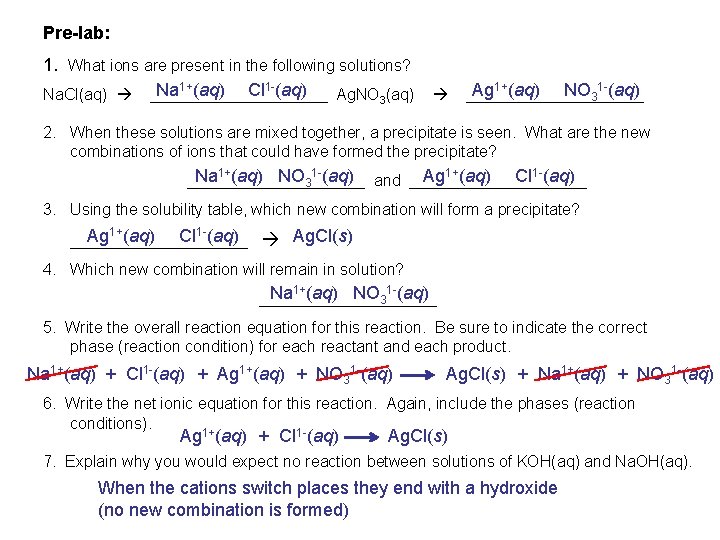
Pre-lab: 1. What ions are present in the following solutions? Na 1+(aq) Cl 1 -(aq) Ag 1+(aq) NO 31 -(aq) Na. Cl(aq) __________ Ag. NO 3(aq) __________ 2. When these solutions are mixed together, a precipitate is seen. What are the new combinations of ions that could have formed the precipitate? Ag (aq) Cl (aq) Na (aq) NO 3 (aq) and ____________________ 1+ 1+ 1 - 1 - 3. Using the solubility table, which new combination will form a precipitate? Ag 1+(aq) Cl 1 -(aq) Ag. Cl(s) __________ 4. Which new combination will remain in solution? Na (aq) NO 3 (aq) __________ 1+ 1 - 5. Write the overall reaction equation for this reaction. Be sure to indicate the correct phase (reaction condition) for each reactant and each product. Na 1+(aq) + Cl 1 -(aq) + Ag 1+(aq) + NO 31 -(aq) Ag. Cl(s) + Na 1+(aq) + NO 31 -(aq) 6. Write the net ionic equation for this reaction. Again, include the phases (reaction conditions). Ag 1+(aq) + Cl 1 -(aq) Ag. Cl(s) 7. Explain why you would expect no reaction between solutions of KOH(aq) and Na. OH(aq). When the cations switch places they end with a hydroxide (no new combination is formed)
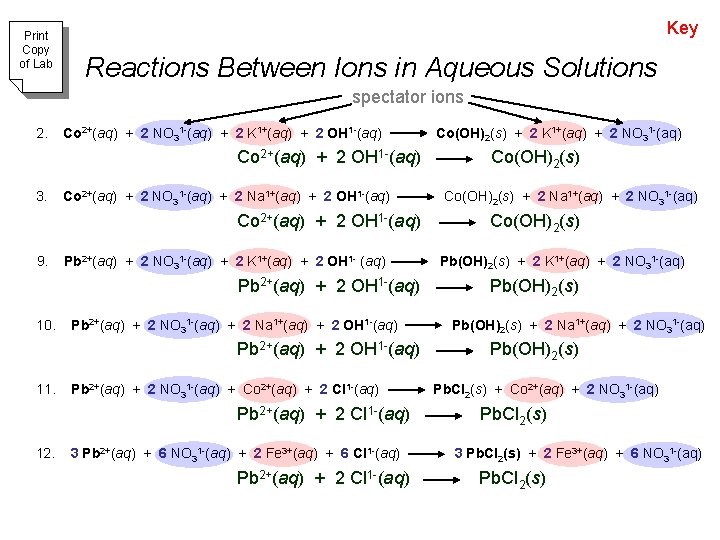
Print Copy of Lab Key Reactions Between Ions in Aqueous Solutions spectator ions 2. Co 2+(aq) + 2 NO 31 -(aq) + 2 K 1+(aq) + 2 OH 1 -(aq) Co 2+(aq) + 2 OH 1 -(aq) 3. Co 2+(aq) + 2 NO 31 -(aq) + 2 Na 1+(aq) + 2 OH 1 -(aq) Co 2+(aq) + 2 OH 1 -(aq) 9. Pb 2+(aq) + 2 NO 31 -(aq) + 2 K 1+(aq) + 2 OH 1 - (aq) Pb 2+(aq) + 2 OH 1 -(aq) 10. Pb 2+(aq) + 2 NO 31 -(aq) + 2 Na 1+(aq) + 2 OH 1 -(aq) Pb 2+(aq) + 2 OH 1 -(aq) 11. Pb 2+(aq) + 2 NO 31 -(aq) + Co 2+(aq) + 2 Cl 1 -(aq) Pb 2+(aq) + 2 Cl 1 -(aq) 12. 3 Pb 2+(aq) + 6 NO 31 -(aq) + 2 Fe 3+(aq) + 6 Cl 1 -(aq) Pb 2+(aq) + 2 Cl 1 -(aq) Co(OH)2(s) + 2 K 1+(aq) + 2 NO 31 -(aq) Co(OH)2(s) + 2 Na 1+(aq) + 2 NO 31 -(aq) Co(OH)2(s) Pb(OH)2(s) + 2 K 1+(aq) + 2 NO 31 -(aq) Pb(OH)2(s) + 2 Na 1+(aq) + 2 NO 31 -(aq) Pb(OH)2(s) Pb. Cl 2(s) + Co 2+(aq) + 2 NO 31 -(aq) Pb. Cl 2(s) 3 Pb. Cl 2(s) + 2 Fe 3+(aq) + 6 NO 31 -(aq) Pb. Cl 2(s)
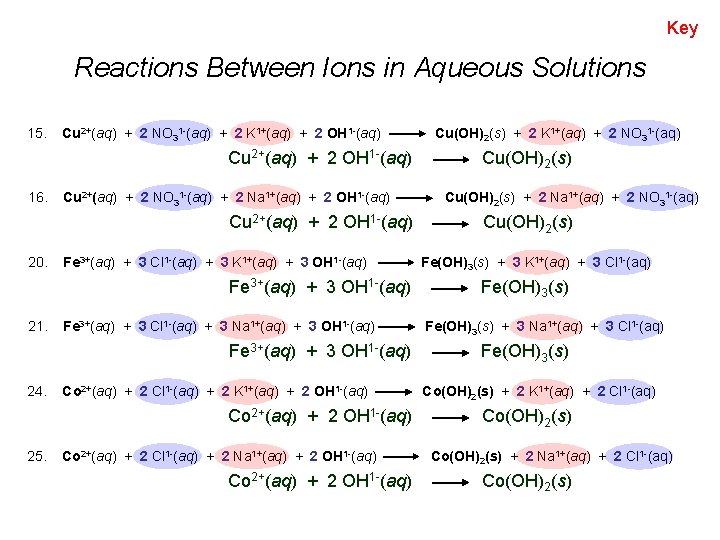
Key Reactions Between Ions in Aqueous Solutions 15. Cu 2+(aq) + 2 NO 31 -(aq) + 2 K 1+(aq) + 2 OH 1 -(aq) Cu 2+(aq) + 2 OH 1 -(aq) 16. Cu 2+(aq) + 2 NO 31 -(aq) + 2 Na 1+(aq) + 2 OH 1 -(aq) Cu 2+(aq) + 2 OH 1 -(aq) 20. Fe 3+(aq) + 3 Cl 1 -(aq) + 3 K 1+(aq) + 3 OH 1 -(aq) Fe 3+(aq) + 3 OH 1 -(aq) 21. Fe 3+(aq) + 3 Cl 1 -(aq) + 3 Na 1+(aq) + 3 OH 1 -(aq) Fe 3+(aq) + 3 OH 1 -(aq) 24. Co 2+(aq) + 2 Cl 1 -(aq) + 2 K 1+(aq) + 2 OH 1 -(aq) Co 2+(aq) + 2 OH 1 -(aq) 25. Co 2+(aq) + 2 Cl 1 -(aq) + 2 Na 1+(aq) + 2 OH 1 -(aq) Co 2+(aq) + 2 OH 1 -(aq) Cu(OH)2(s) + 2 K 1+(aq) + 2 NO 31 -(aq) Cu(OH)2(s) + 2 Na 1+(aq) + 2 NO 31 -(aq) Cu(OH)2(s) Fe(OH)3(s) + 3 K 1+(aq) + 3 Cl 1 -(aq) Fe(OH)3(s) + 3 Na 1+(aq) + 3 Cl 1 -(aq) Fe(OH)3(s) Co(OH)2(s) + 2 K 1+(aq) + 2 Cl 1 -(aq) Co(OH)2(s) + 2 Na 1+(aq) + 2 Cl 1 -(aq) Co(OH)2(s)
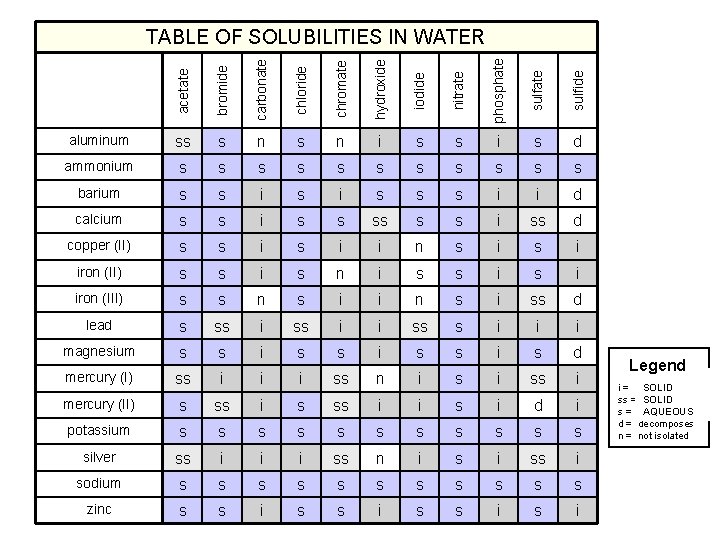
acetate bromide carbonate chloride chromate hydroxide iodide nitrate phosphate sulfide TABLE OF SOLUBILITIES IN WATER aluminum ss s n i s s i s d ammonium s s s barium s s i s s s i i d calcium s s i ss d copper (II) s s i i n s i iron (II) s s i s n i s s i iron (III) s s n s i i n s i ss d lead s ss i i ss s i i i magnesium s s i s d mercury (I) ss i i i ss n i ss i mercury (II) s ss i i s i d i potassium s s silver ss i i i ss n i ss i sodium s s s zinc s s i Legend SOLID i = insoluble SOLIDsoluble ss = slightly AQUEOUS s = soluble d = decomposes n = not isolated
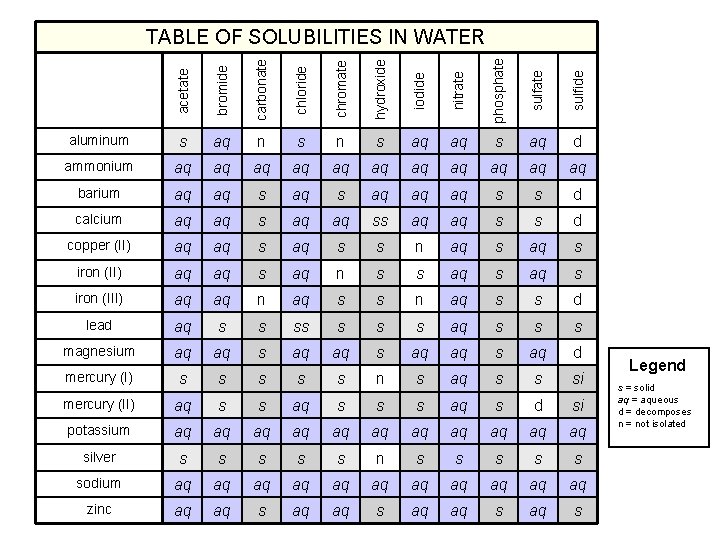
acetate bromide carbonate chloride chromate hydroxide iodide nitrate phosphate sulfide TABLE OF SOLUBILITIES IN WATER aluminum s aq n s aq aq s aq d ammonium aq aq aq barium aq aq s aq aq aq s s d calcium aq aq ss aq aq s s d copper (II) aq aq s s n aq s iron (II) aq aq s aq n s s aq s iron (III) aq aq n aq s s d lead aq s s s s aq s s s magnesium aq aq s aq d mercury (I) s s s n s aq s s si mercury (II) aq s s s aq s d si potassium aq aq aq silver s s s n s s sodium aq aq aq zinc aq aq s Legend s = solid aq = aqueous d = decomposes n = not isolated
- Slides: 8