Ions Alchemy Lesson V Chemistry Opener Chem Survey






- Slides: 21

Ions Alchemy Lesson V Chemistry

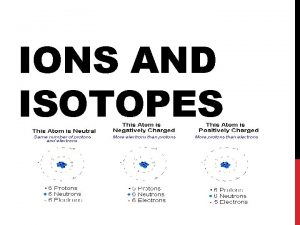
Opener: Chem Survey ¨ We’ve learned that, although difficult, an atom has the ability to accommodate changes in its nucleus. By losing or absorbing nuclear particles, an atom can completely change its identity! Do you think it’s possible for an atom to gain or lose electrons? Do you think it is an easier or more difficult process? Explain.

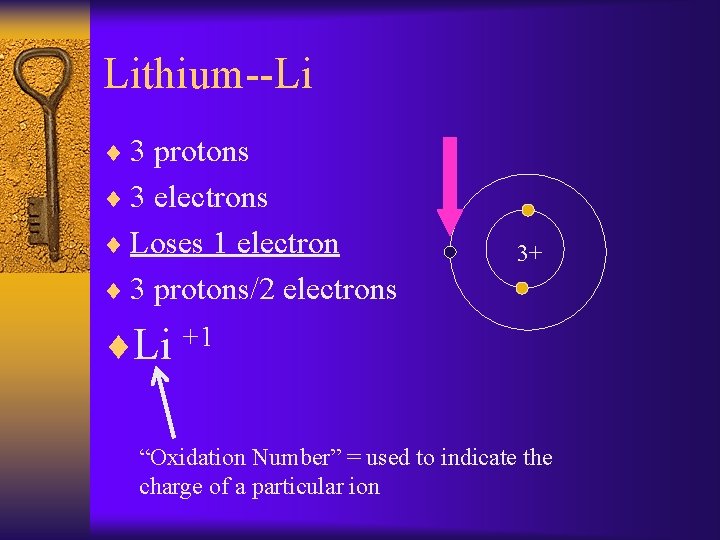
Lithium--Li ¨ 3 protons ¨ 3 electrons ¨ Loses 1 electron 3+ ¨ 3 protons/2 electrons ¨Li +1 “Oxidation Number” = used to indicate the charge of a particular ion

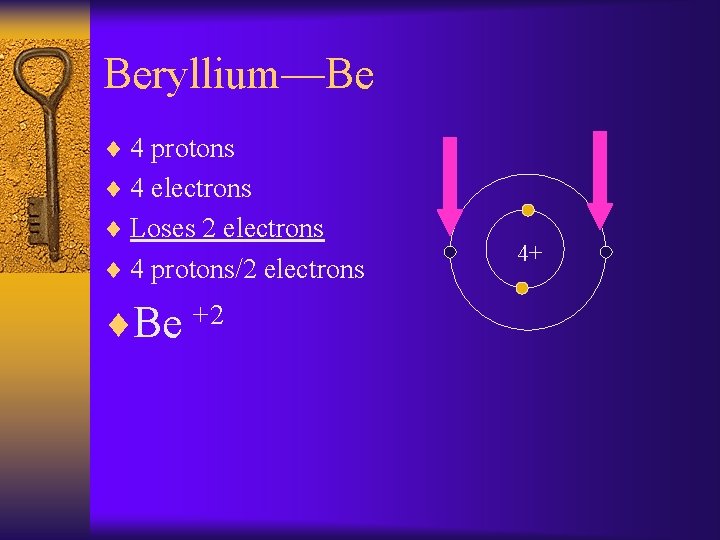
Beryllium—Be ¨ 4 protons ¨ 4 electrons ¨ Loses 2 electrons ¨ 4 protons/2 electrons ¨Be +2 4+

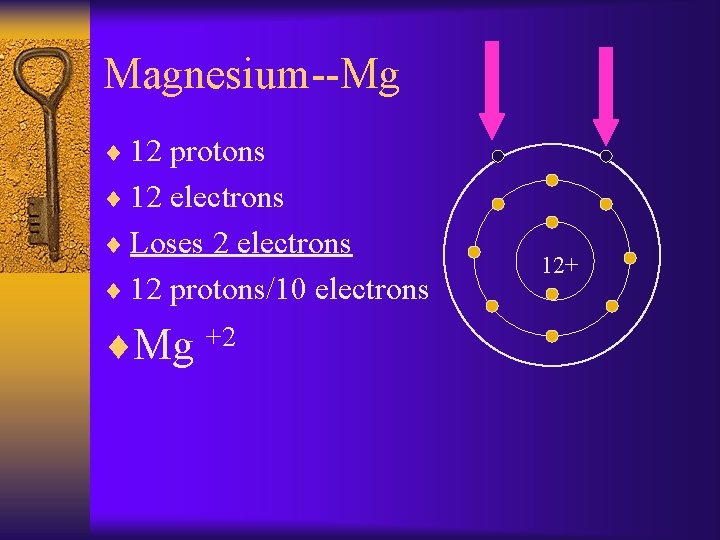
Magnesium--Mg ¨ 12 protons ¨ 12 electrons ¨ Loses 2 electrons ¨ 12 protons/10 electrons ¨Mg +2 12+

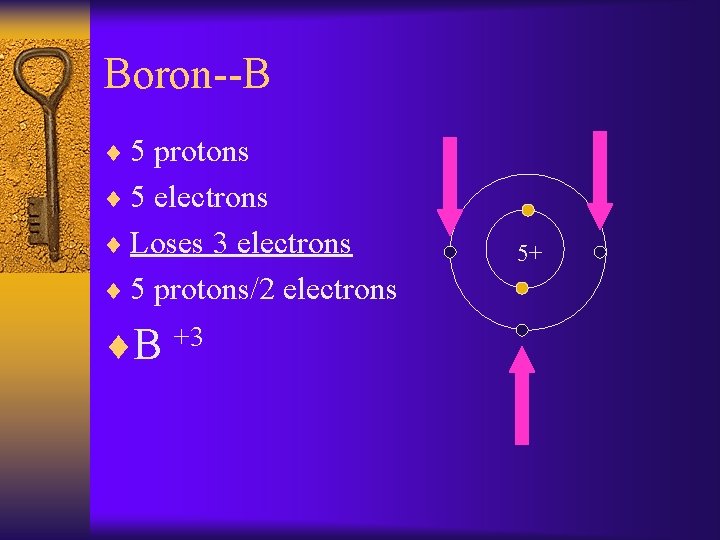
Boron--B ¨ 5 protons ¨ 5 electrons ¨ Loses 3 electrons ¨ 5 protons/2 electrons ¨B +3 5+

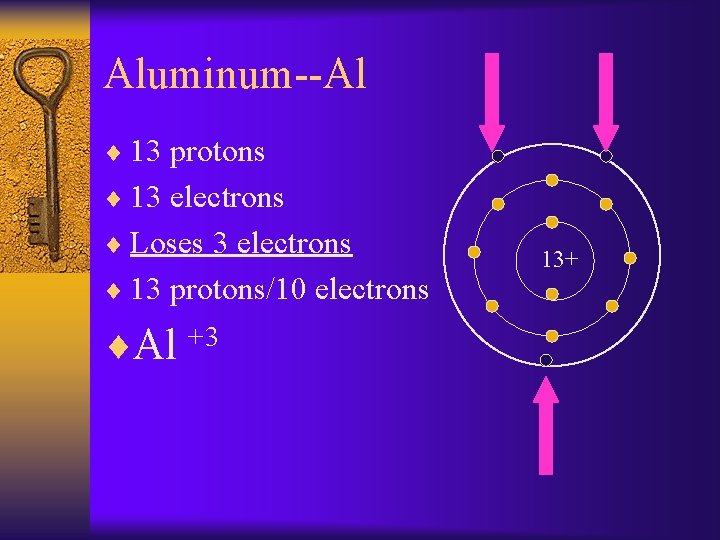
Aluminum--Al ¨ 13 protons ¨ 13 electrons ¨ Loses 3 electrons ¨ 13 protons/10 electrons ¨Al +3 13+

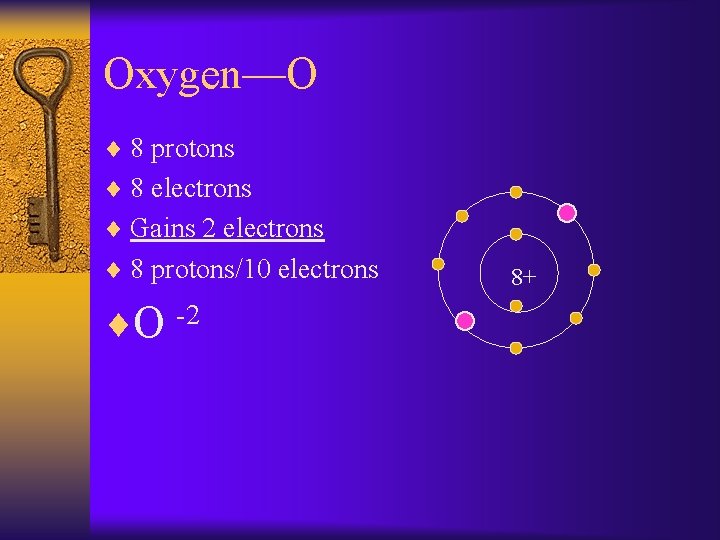
Oxygen—O ¨ 8 protons ¨ 8 electrons ¨ Gains 2 electrons ¨ 8 protons/10 electrons ¨O -2 8+

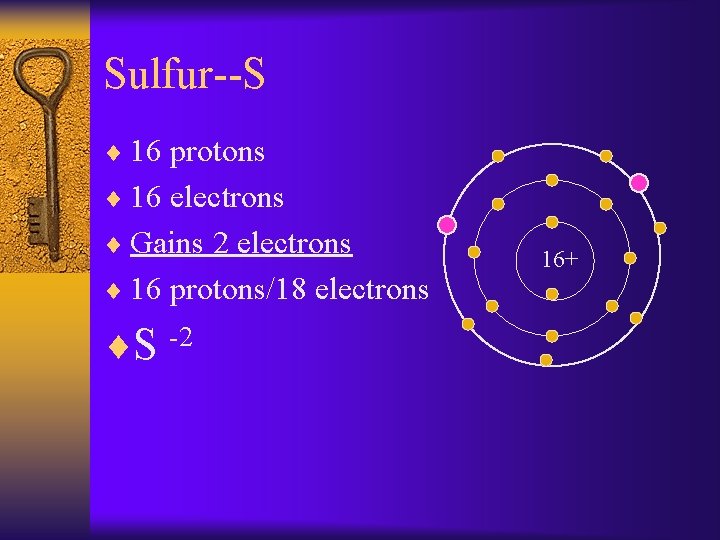
Sulfur--S ¨ 16 protons ¨ 16 electrons ¨ Gains 2 electrons ¨ 16 protons/18 electrons ¨S -2 16+

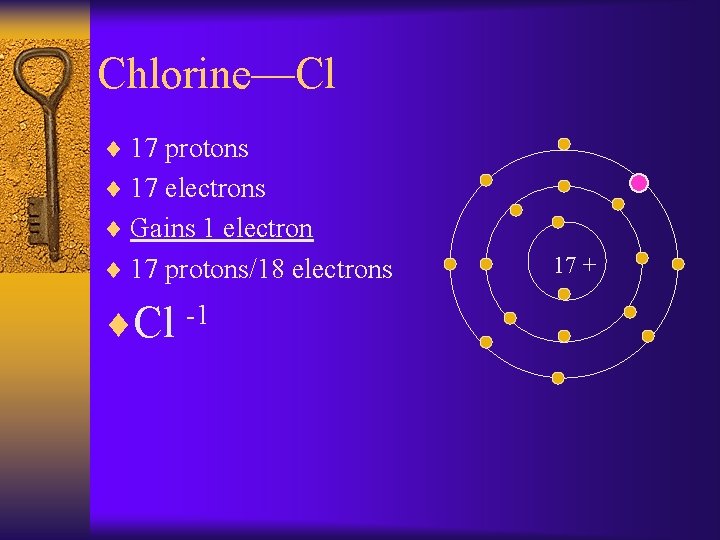
Chlorine—Cl ¨ 17 protons ¨ 17 electrons ¨ Gains 1 electron ¨ 17 protons/18 electrons ¨Cl -1 17 +

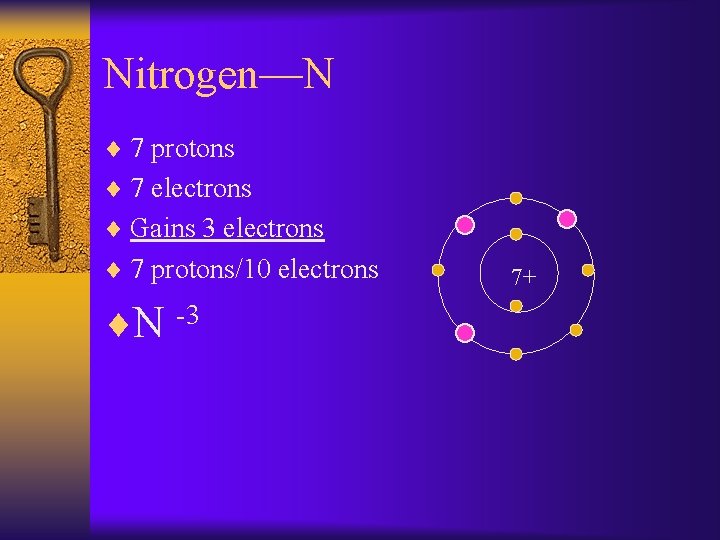
Nitrogen—N ¨ 7 protons ¨ 7 electrons ¨ Gains 3 electrons ¨ 7 protons/10 electrons ¨N -3 7+

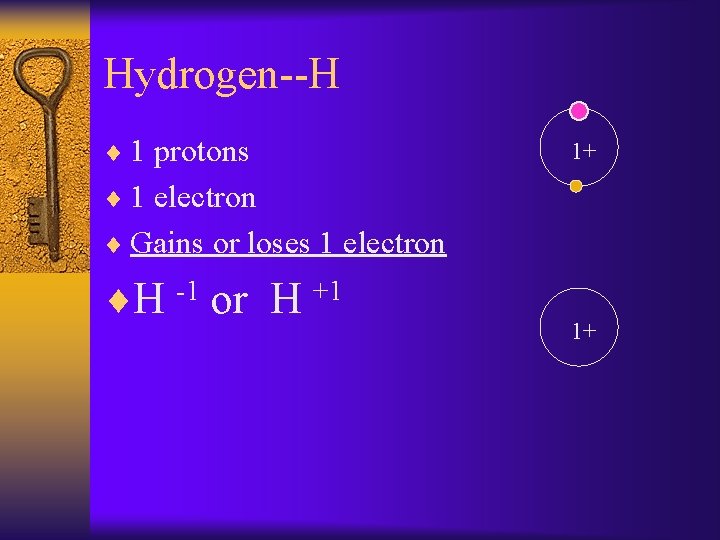
Hydrogen--H ¨ 1 protons 1+ ¨ 1 electron ¨ Gains or loses 1 electron ¨H -1 or H +1 1+

How do you know…+ or -? ¨ All atoms have “noble gas envy”. ¨ Electronically speaking, they want to be like a noble gas – having 8 valence electrons. ¨ Atoms will either gain or lose electrons based on how ‘close’ they are to a noble gas. ¨ Ex: Oxygen has 6 valence electrons – do you suppose it is ‘easier’ for O to gain 2, or lose 6 electrons?

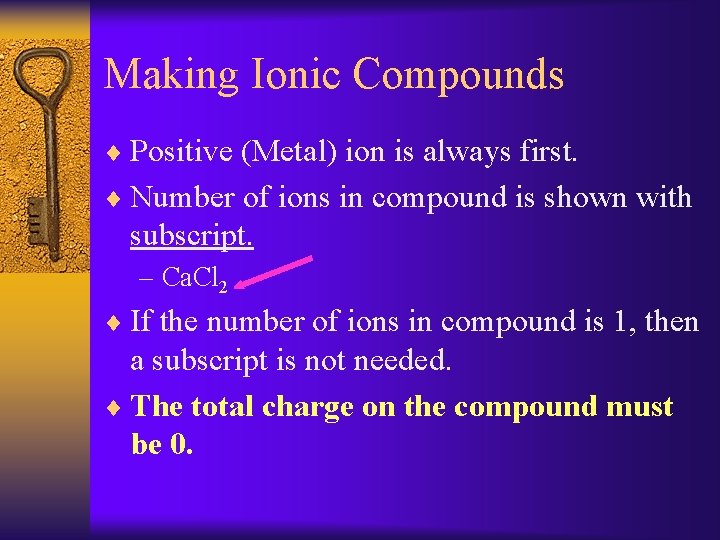
Making Ionic Compounds ¨ Positive (Metal) ion is always first. ¨ Number of ions in compound is shown with subscript. – Ca. Cl 2 ¨ If the number of ions in compound is 1, then a subscript is not needed. ¨ The total charge on the compound must be 0.

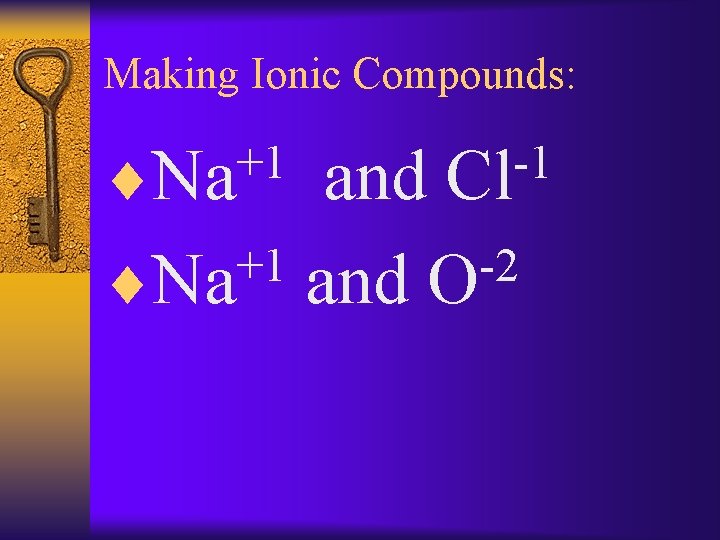
Making Ionic Compounds: +1 ¨Na -1 Cl and +1 -2 ¨Na and O

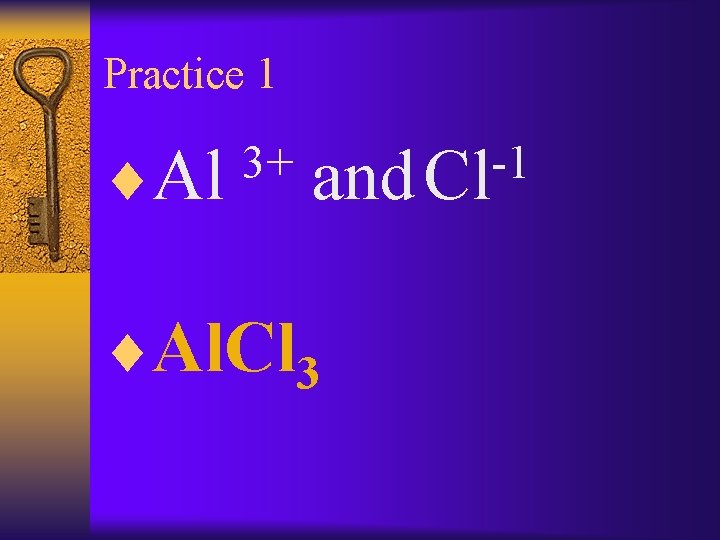
Practice 1 ¨Al 3+ -1 and Cl ¨Al. Cl 3

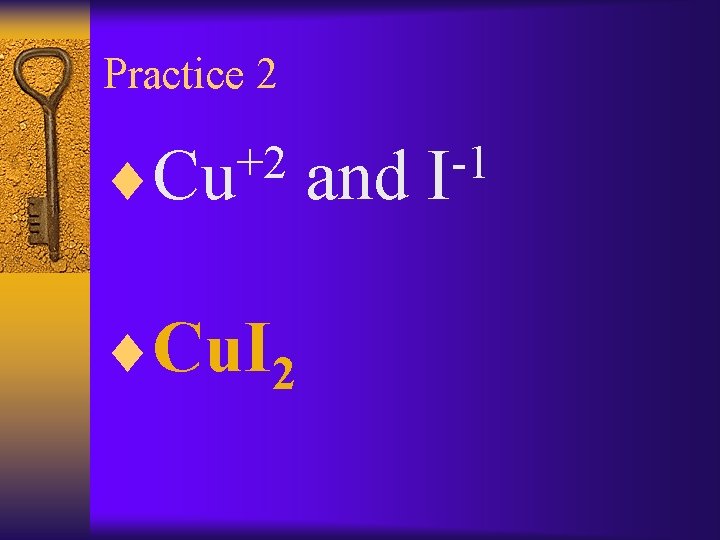
Practice 2 +2 ¨Cu. I 2 and -1 I

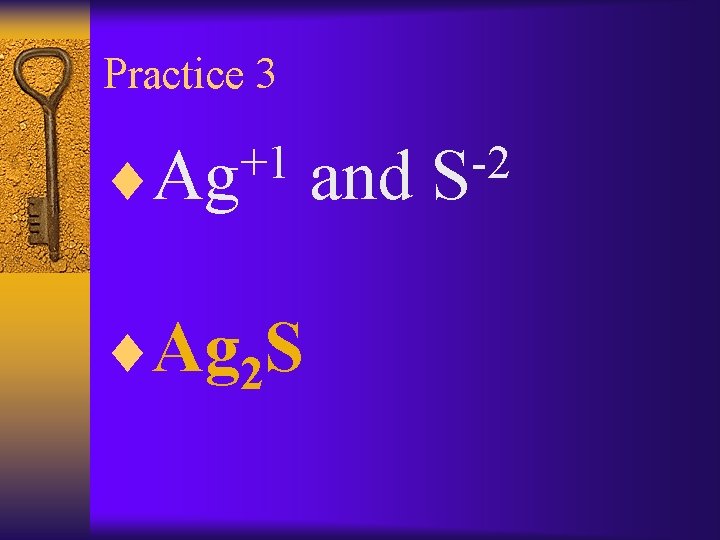
Practice 3 +1 ¨Ag 2 S and -2 S

Practice 4 2+ ¨Mg and -2 S ¨Mg. S (simplify!!)

Practice 5: For Experts!!! +3 ¨Fe and ¨Fe 2 O 3 -2 O

OK, What’s the Trick? ?
 What is alchemy in chemistry
What is alchemy in chemistry Chemist and alchemist
Chemist and alchemist Better tm
Better tm What do the roman numerals in a cation's name indicate?
What do the roman numerals in a cation's name indicate? Lesson 20 getting connected ionic compounds
Lesson 20 getting connected ionic compounds Polyatomic cation
Polyatomic cation Spectator ions in chemistry
Spectator ions in chemistry Abcd in acls
Abcd in acls Lesson 19 noble gas envy ions answers
Lesson 19 noble gas envy ions answers Ed ing ly openers
Ed ing ly openers Appositive list
Appositive list Ing sentence opener
Ing sentence opener Adverb opener examples
Adverb opener examples Participial and prepositional phrases
Participial and prepositional phrases Whats an appositive
Whats an appositive Rfi filter for garage door opener
Rfi filter for garage door opener Types of sentence openers
Types of sentence openers Lever simple machines
Lever simple machines Whats a participial phrase
Whats a participial phrase Can opener
Can opener Forcible door entry device factory
Forcible door entry device factory Balance opener
Balance opener