Ionization techniques Mass spectra and MSInstrumentation Molecule ionization

![Number of C = 100×[M+1]/([M]× 1. 1) Number of C = 100×[M+1]/([M]× 1. 1)](https://slidetodoc.com/presentation_image_h2/9662a326e351c4745d70e28cb4e360a8/image-14.jpg)



- Slides: 32

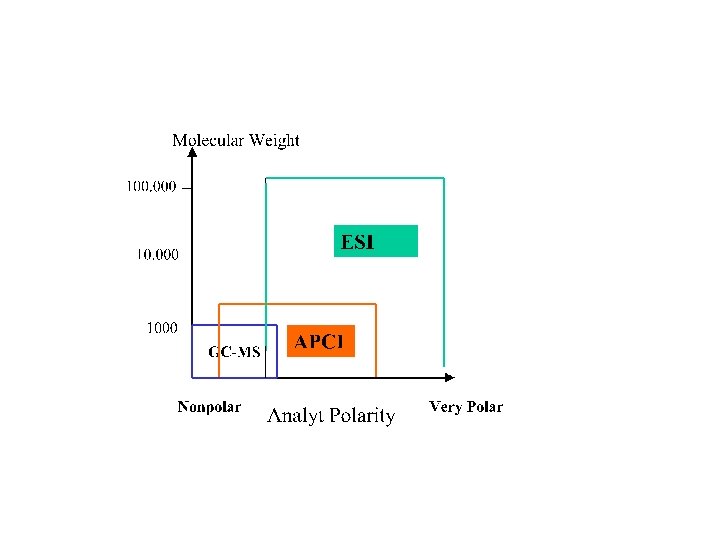
Ionization techniques, Mass spectra and MS-Instrumentation Molecule ionization methods: 1. Electron impact (EI) and Chemical ionization (CE) (GC-MS) 2. Electro spray ionization (ESI) (LC-ESI-MS) 3. Matrix assisted laser desorption (MALDI)


Agilent GC-MS


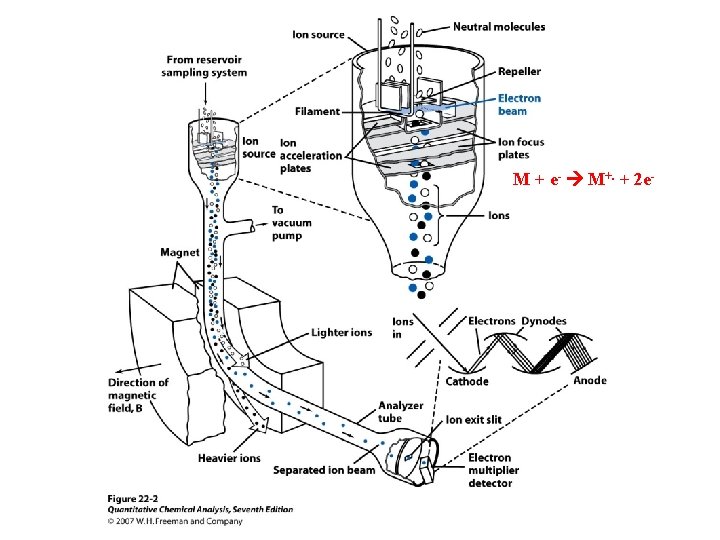
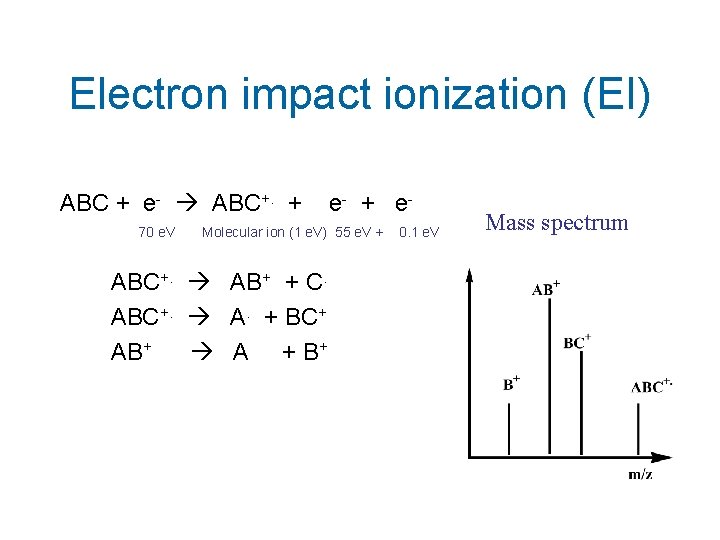
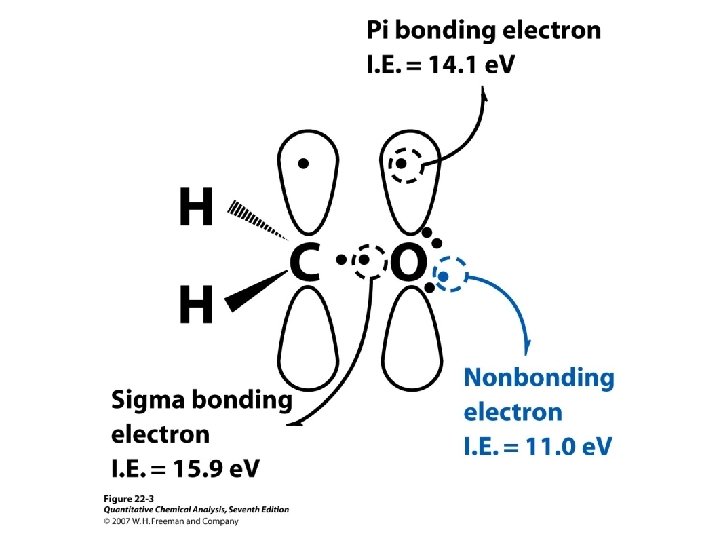
Electron impact ionization (EI) ABC + e- ABC+. + 70 e. V e- + e- Molecular ion (1 e. V) 55 e. V + ABC+. AB+ + C. ABC+. A. + BC+ AB+ A + B+ 0. 1 e. V Mass spectrum

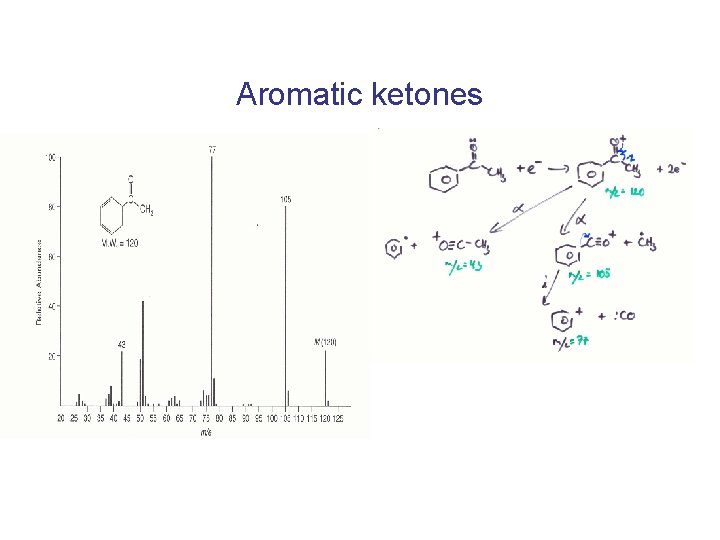
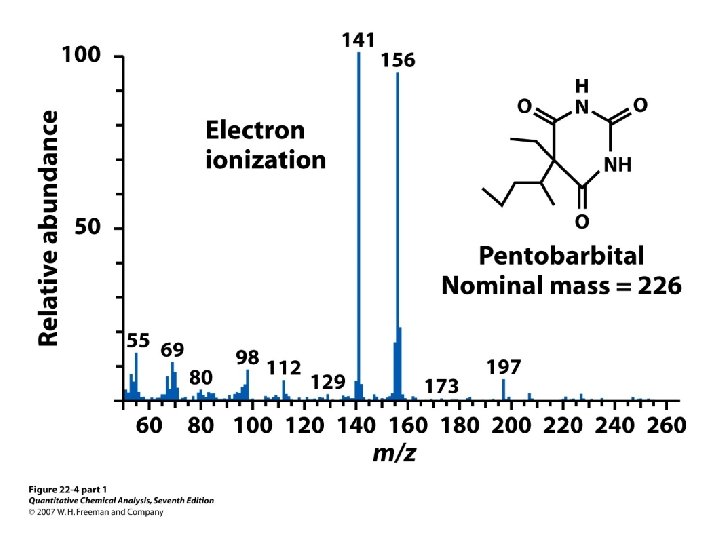
Aromatic ketones


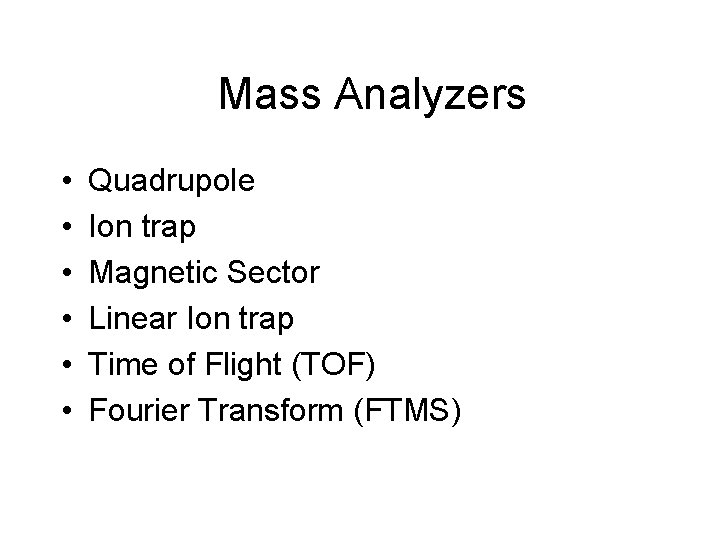
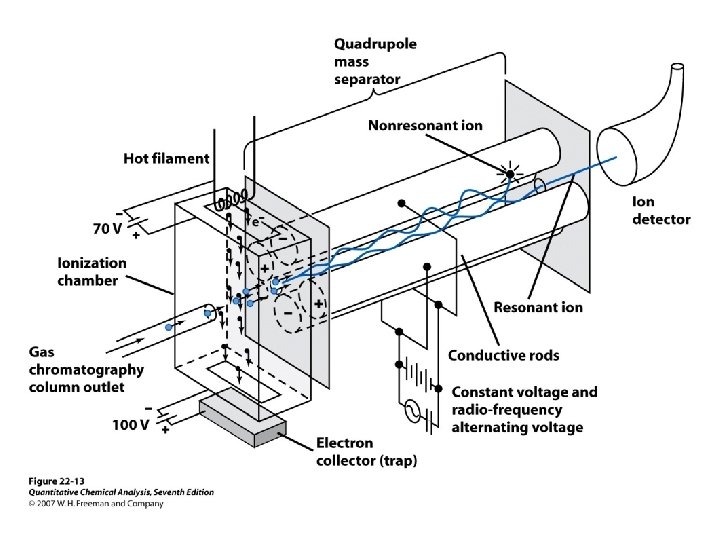
Mass Analyzers • • • Quadrupole Ion trap Magnetic Sector Linear Ion trap Time of Flight (TOF) Fourier Transform (FTMS)



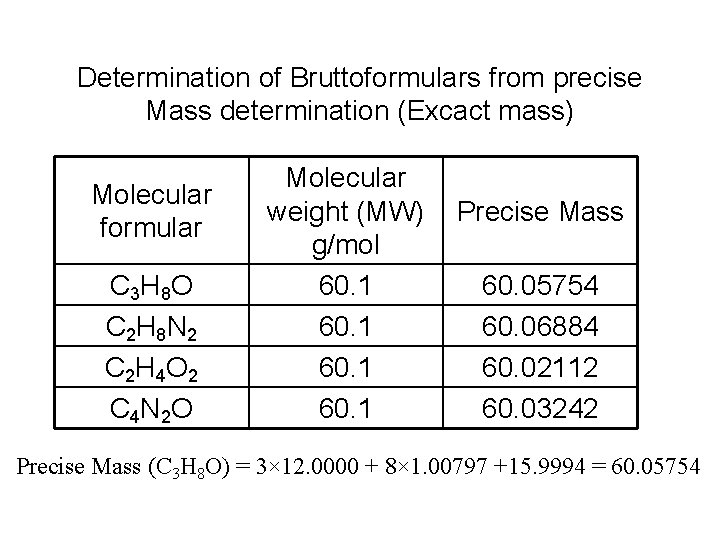
Determination of Bruttoformulars from precise Mass determination (Excact mass) Molecular formular C 3 H 8 O C 2 H 8 N 2 C 2 H 4 O 2 C 4 N 2 O Molecular weight (MW) g/mol 60. 1 Precise Mass 60. 05754 60. 06884 60. 02112 60. 03242 Precise Mass (C 3 H 8 O) = 3× 12. 0000 + 8× 1. 00797 +15. 9994 = 60. 05754

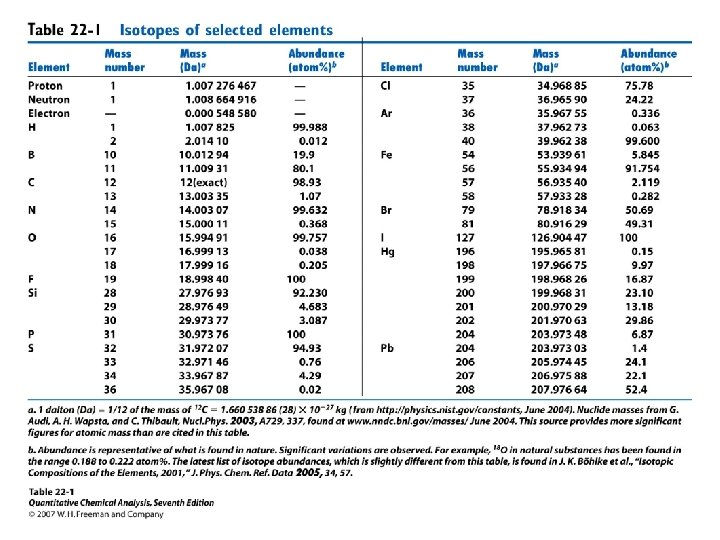
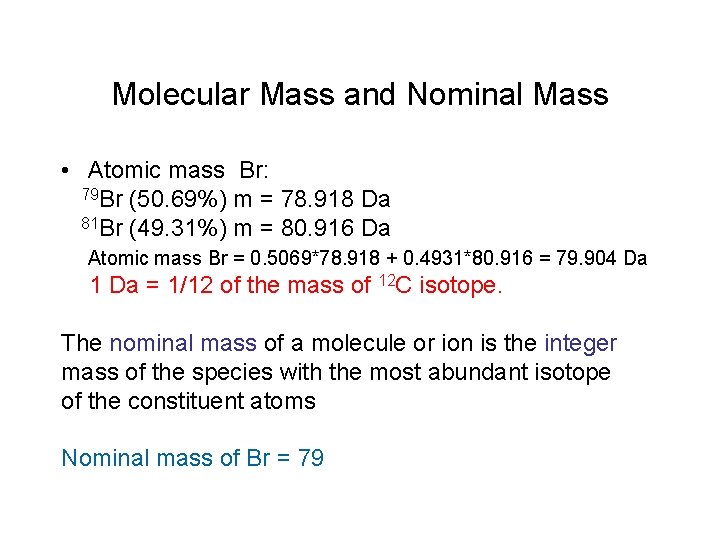
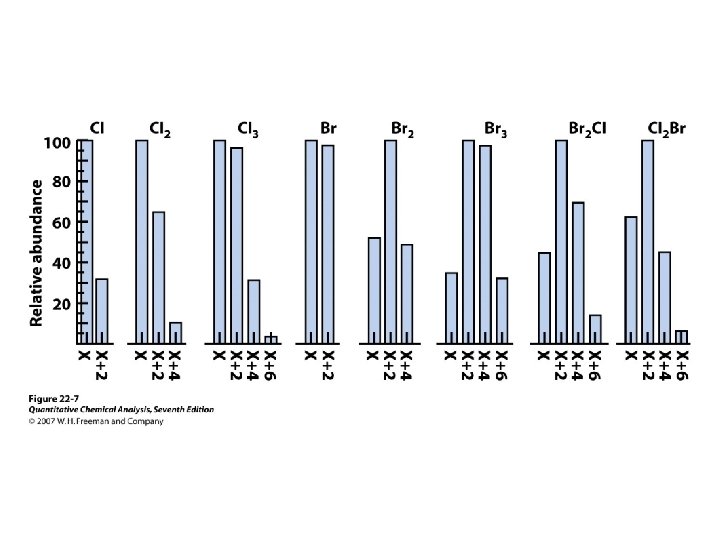
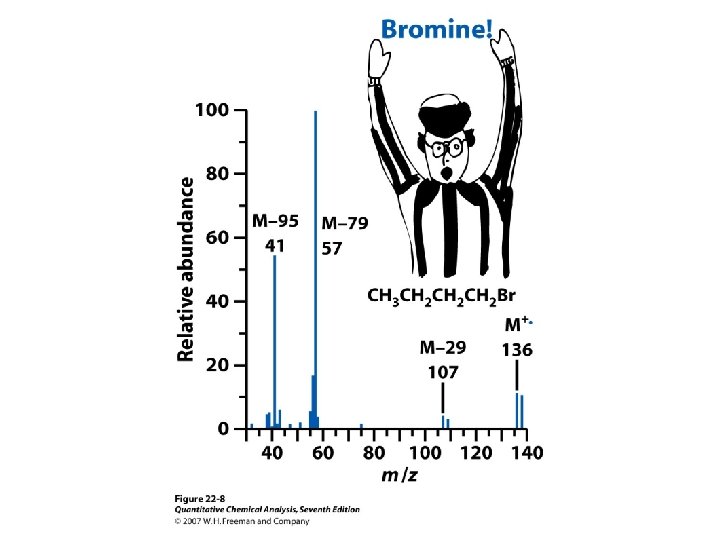
Molecular Mass and Nominal Mass • Atomic mass Br: 79 Br (50. 69%) m = 78. 918 Da 81 Br (49. 31%) m = 80. 916 Da Atomic mass Br = 0. 5069*78. 918 + 0. 4931*80. 916 = 79. 904 Da 1 Da = 1/12 of the mass of 12 C isotope. The nominal mass of a molecule or ion is the integer mass of the species with the most abundant isotope of the constituent atoms Nominal mass of Br = 79

![Number of C 100M1M 1 1 Number of C = 100×[M+1]/([M]× 1. 1)](https://slidetodoc.com/presentation_image_h2/9662a326e351c4745d70e28cb4e360a8/image-14.jpg)
Number of C = 100×[M+1]/([M]× 1. 1)



Nitrogen rule • If a compound contains an even number (0, 2, 4…) of nitrogen atoms, its molecular ion will be an even mass number. • An odd number (1, 3, 5. . ) of nitrogen atoms => The mass number of M+. will be odd.

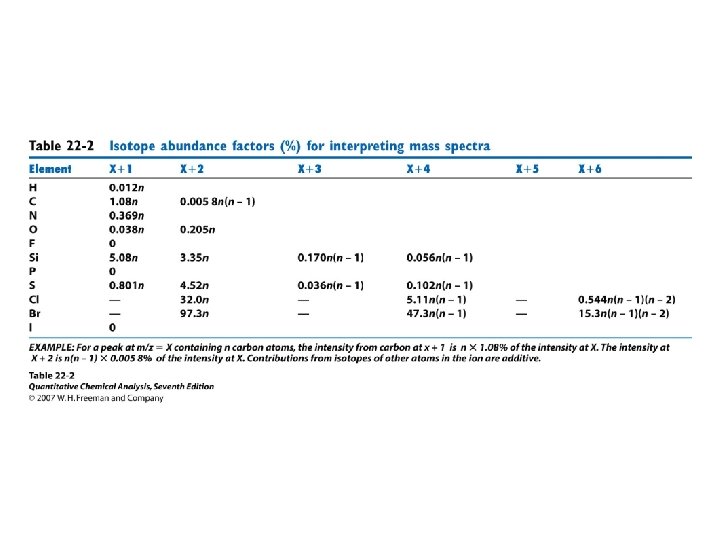
Basic advices 1) 2) 3) 4) 5) 6) Identify the mole peak M+. Nitogen rule: Determine number of N A+2 elements: Cl, Br, S, Si NC = 100×[M+1]/([M]× 1. 1) Determine brutto formular Calculate r + db = NC – NH/2 + NN/2 + 1 (halogens counts as H)

Stevensons rule • The most probable fragmentation is the one that leaves the positive charge on the fragment with the lowest ionization energy • H 3 C+ < RCH 2+ < R 2 CH+ < H 2 C=CH-CH 2+ < C 6 H 6 CH 2+ Difficult Easy


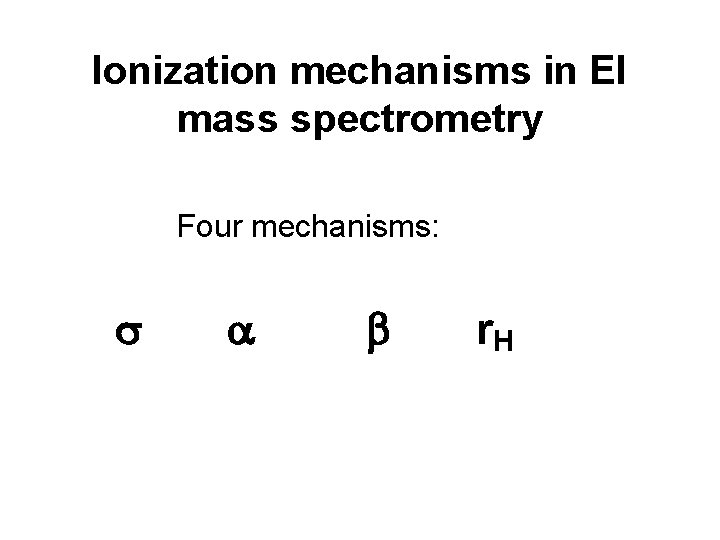
Ionization mechanisms in EI mass spectrometry Four mechanisms: s a b r. H

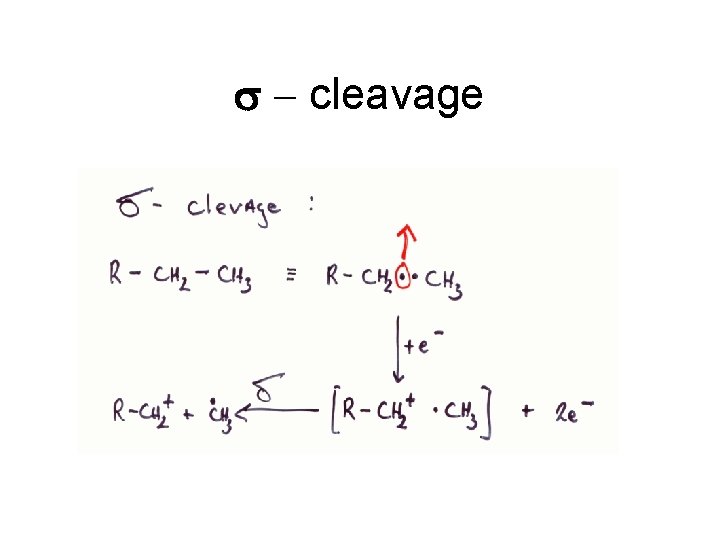
s - cleavage

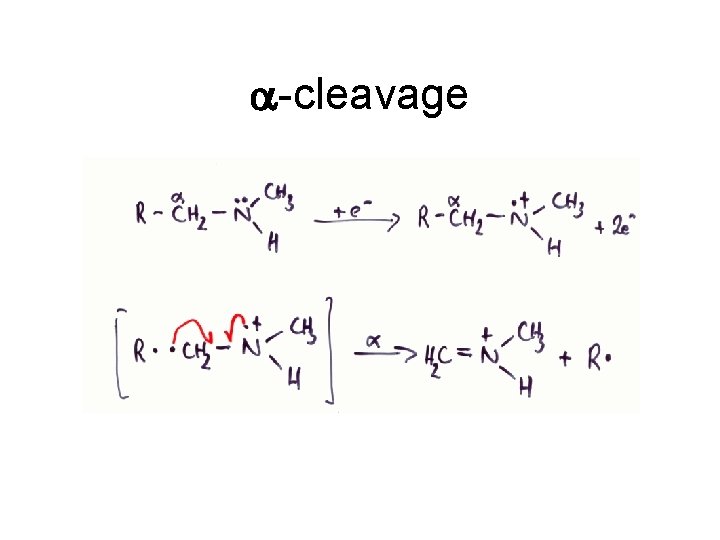
a-cleavage

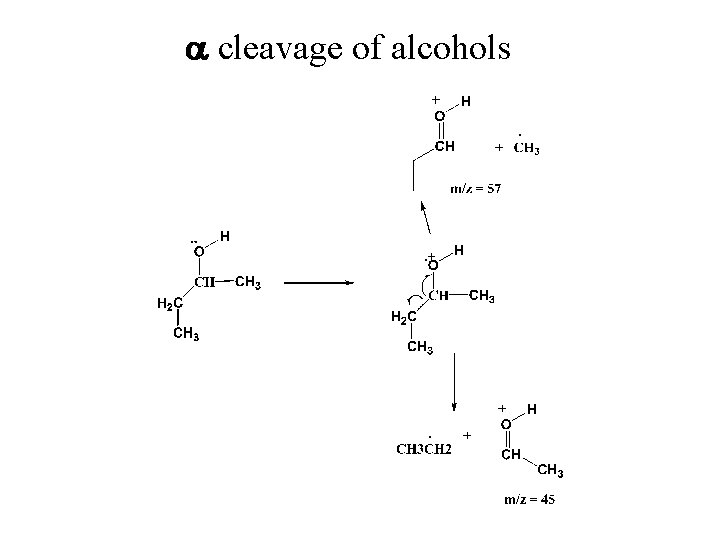
a cleavage of alcohols

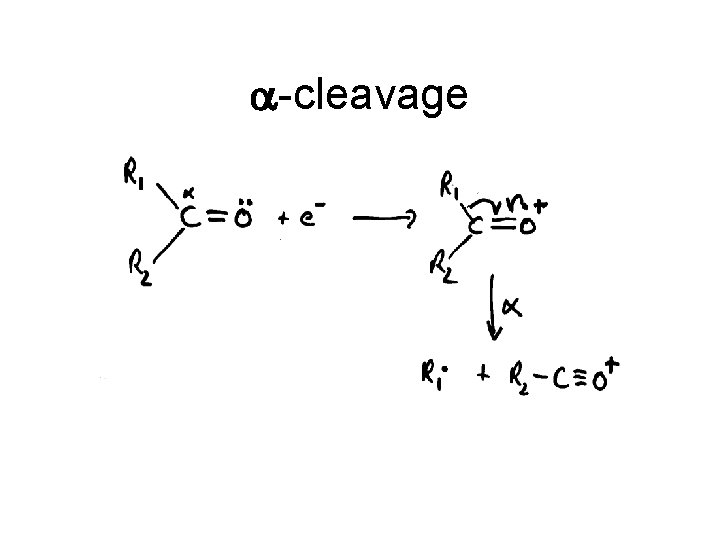
a-cleavage

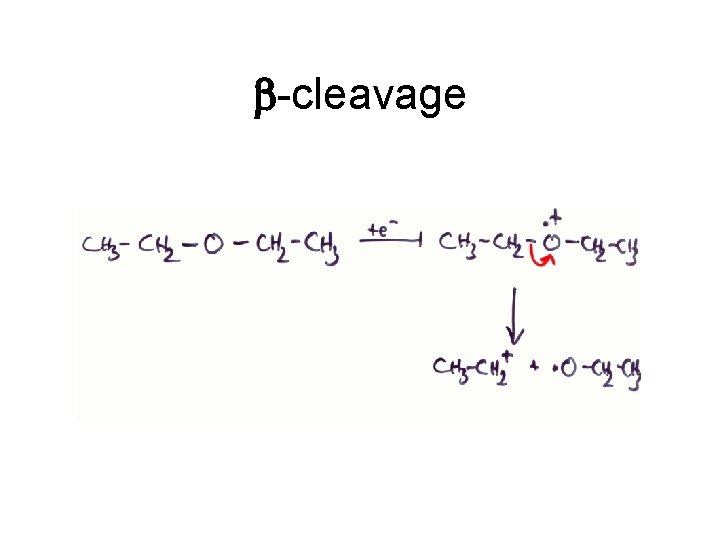
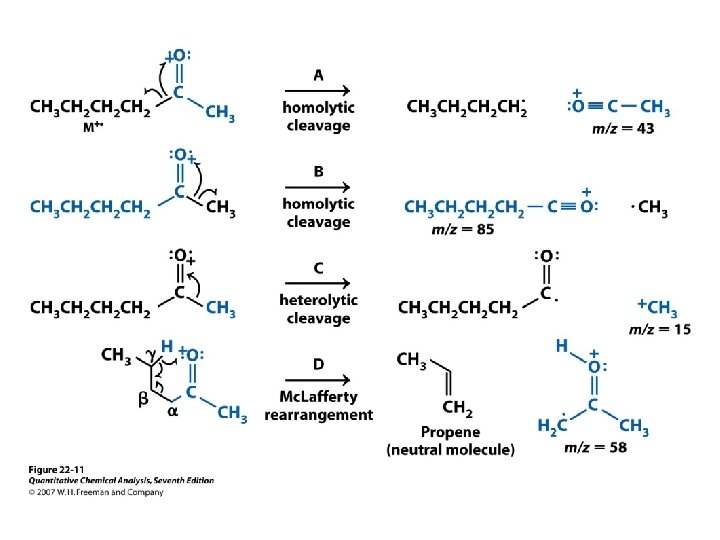
b-cleavage

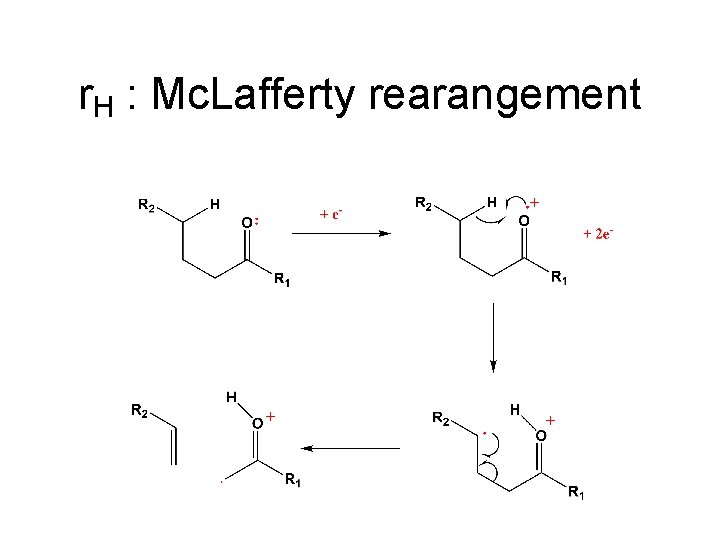
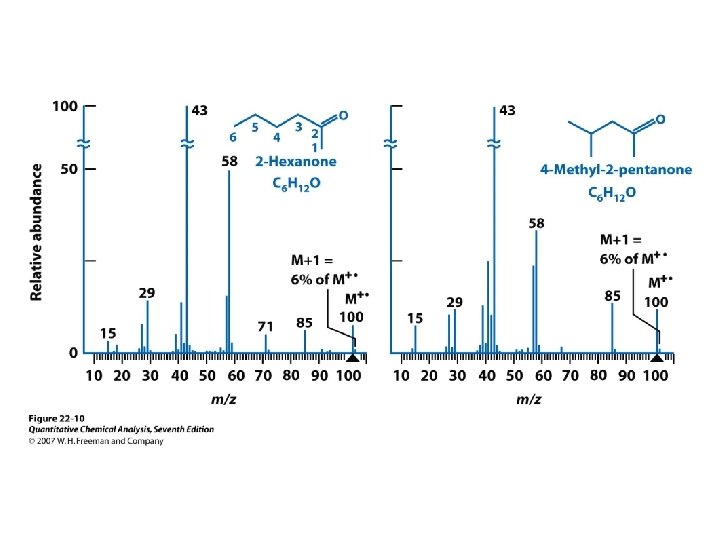
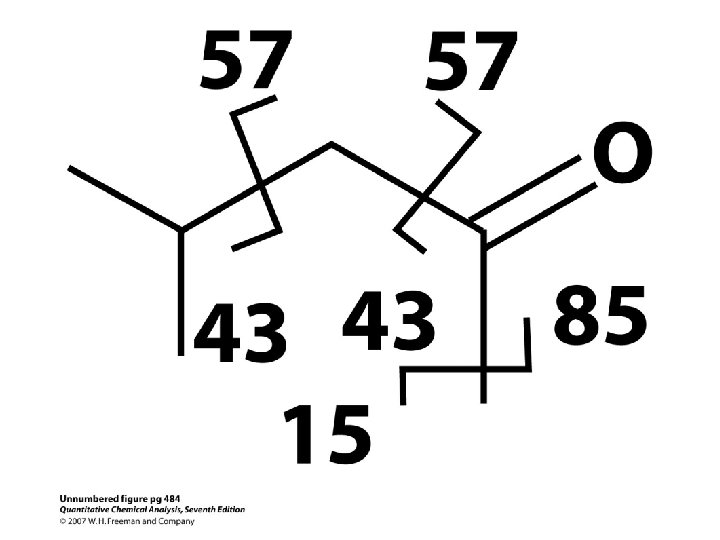
r. H : Mc. Lafferty rearangement




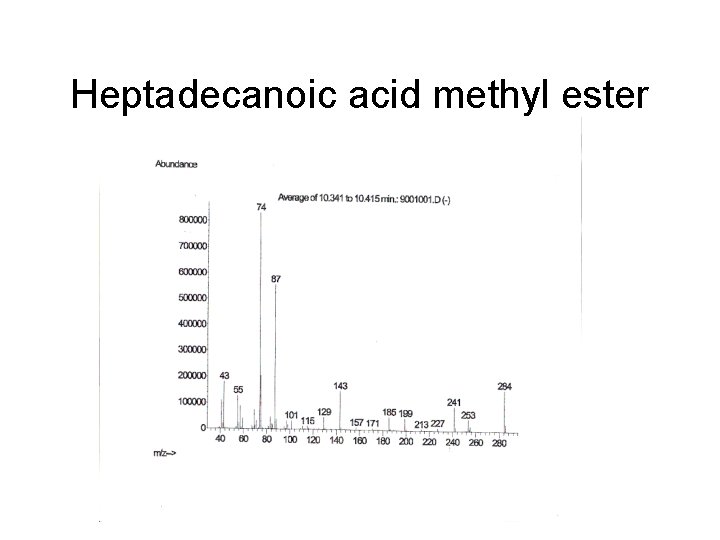
Heptadecanoic acid methyl ester

Ionization techniques, Mass spectra and MS-Instrumentation Molecule ionization methods: 1. 2. Electron impact (EI) and Chemical ionization (CE) (GC-MS) Electro spray ionization (ESI) (LC-ESI-MS)