Ionization Constant of Water and p H Water



![Kw= + [H ][OH ] It has a special name. The ionization constant of Kw= + [H ][OH ] It has a special name. The ionization constant of](https://slidetodoc.com/presentation_image_h2/bcdba7c8c698672cc514e0d9f926072c/image-4.jpg)
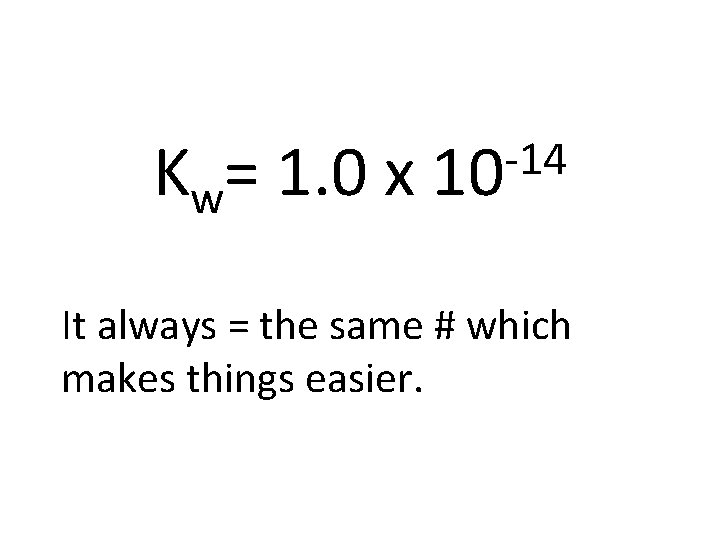
![1. 0 x -14 10 = + [H ][OH ] So if you know 1. 0 x -14 10 = + [H ][OH ] So if you know](https://slidetodoc.com/presentation_image_h2/bcdba7c8c698672cc514e0d9f926072c/image-6.jpg)
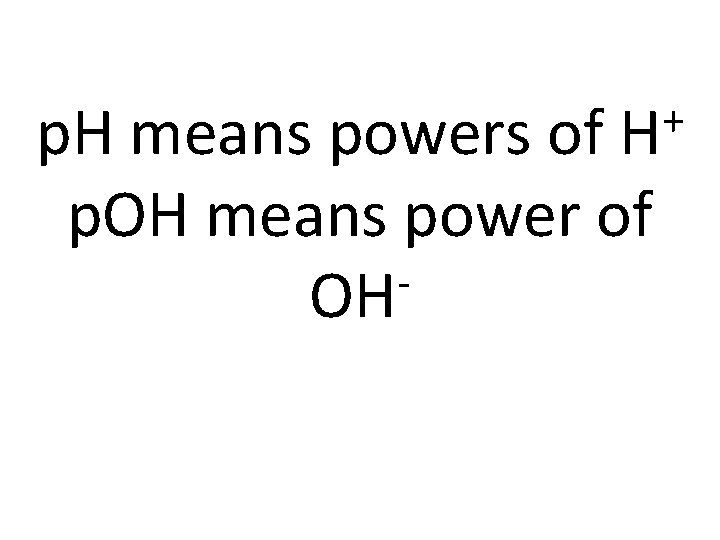
![-14 10 = + [H ][OH ] 1. 0 x -log of everything = -14 10 = + [H ][OH ] 1. 0 x -log of everything =](https://slidetodoc.com/presentation_image_h2/bcdba7c8c698672cc514e0d9f926072c/image-10.jpg)





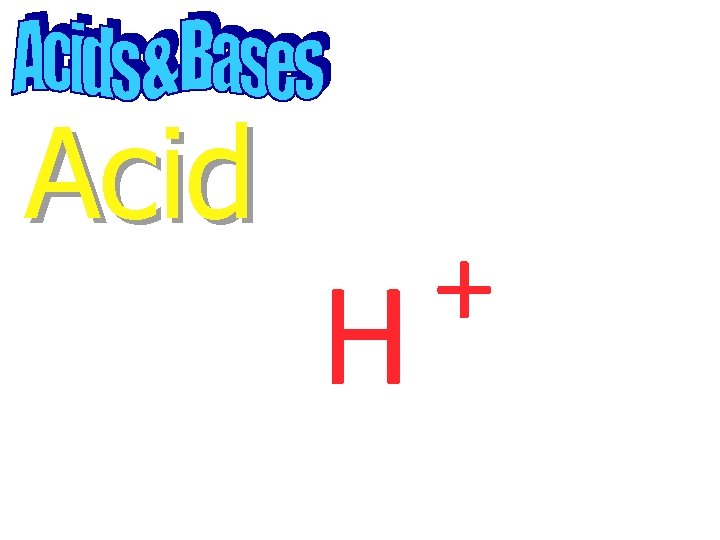










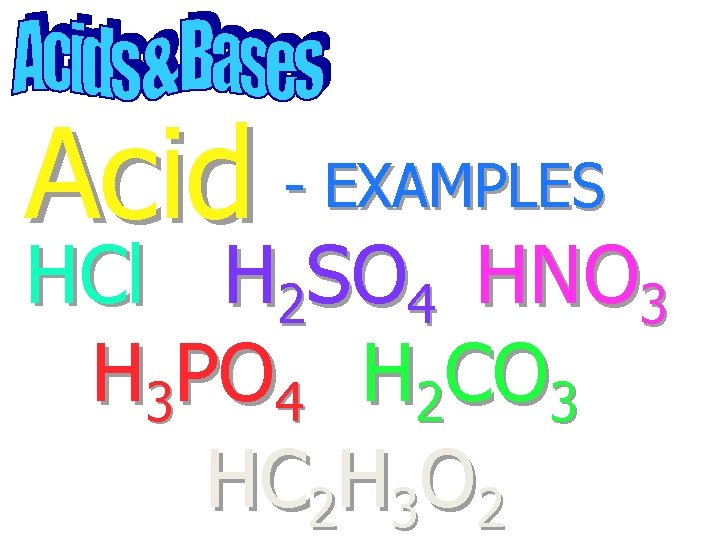



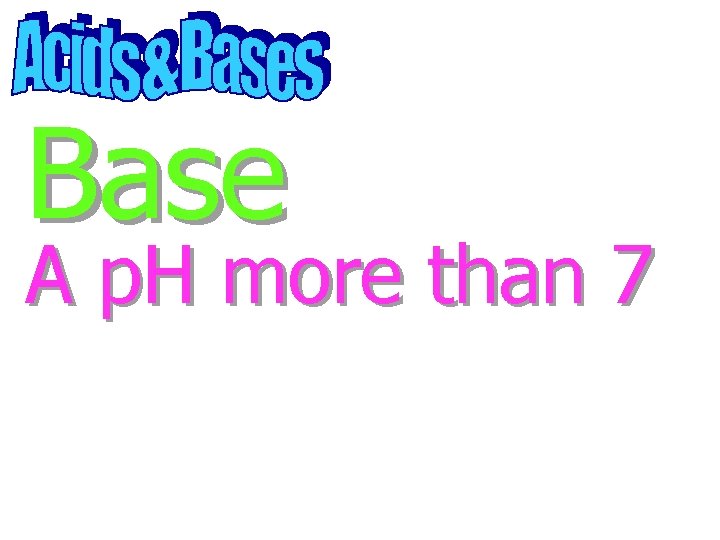













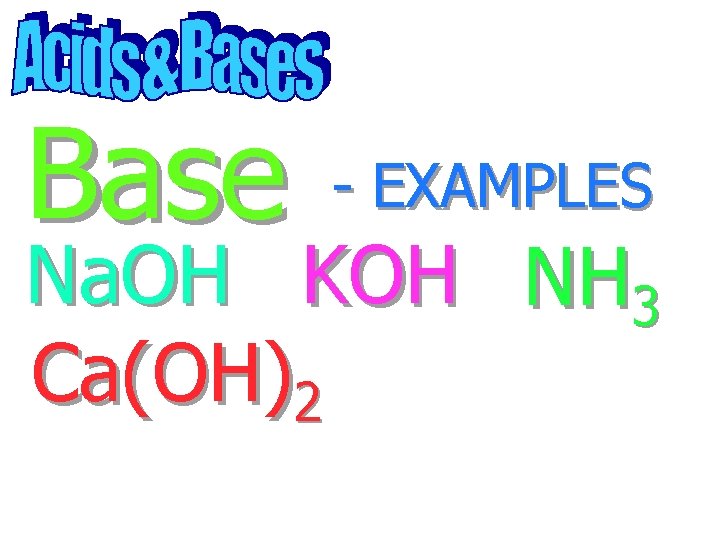
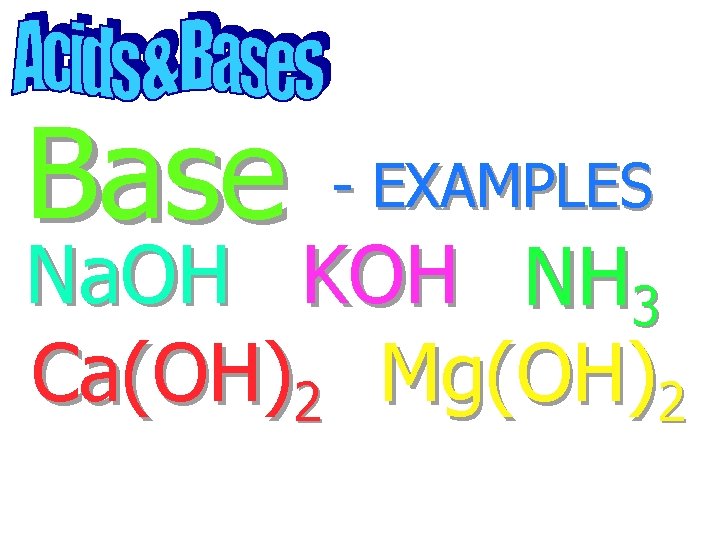
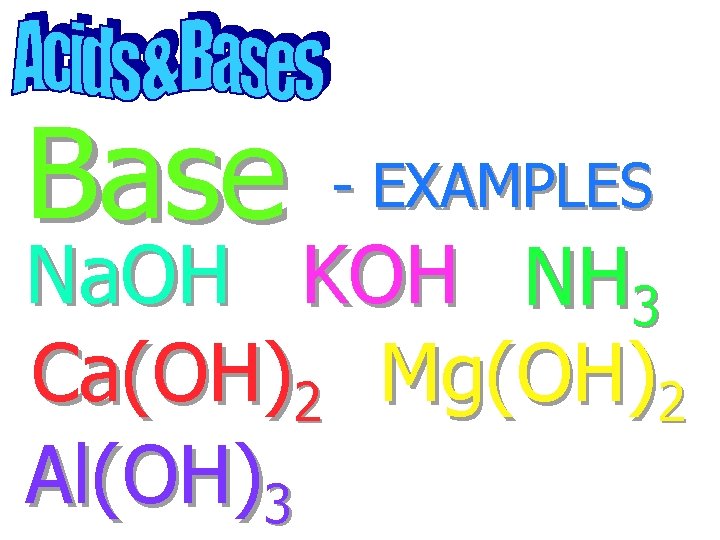
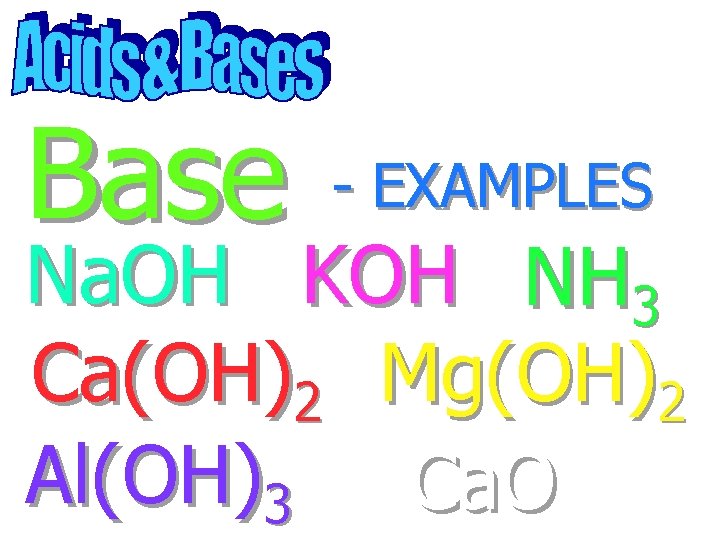



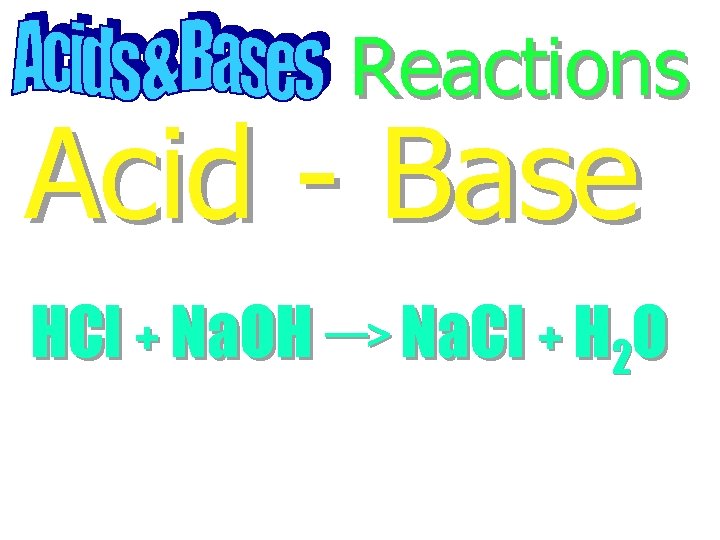

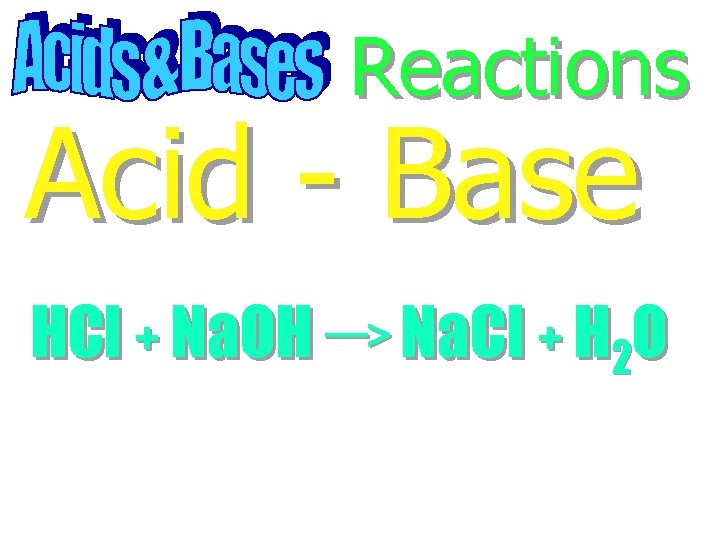
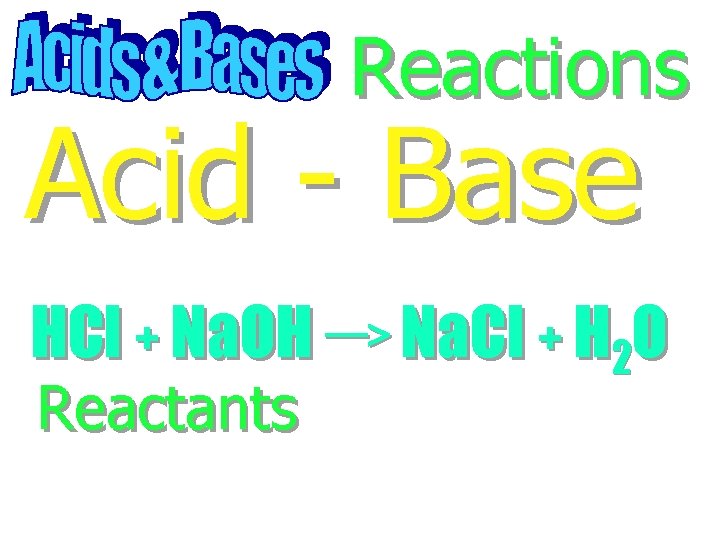



- Slides: 63

Ionization Constant of Water and p. H

Water breaks apart into two things + Hydrogen – H Hydroxide – OH

H 2 O(l) + H (aq) + OH Write the equilibrium expression for this equation. (aq)
![Kw H OH It has a special name The ionization constant of Kw= + [H ][OH ] It has a special name. The ionization constant of](https://slidetodoc.com/presentation_image_h2/bcdba7c8c698672cc514e0d9f926072c/image-4.jpg)
Kw= + [H ][OH ] It has a special name. The ionization constant of water.
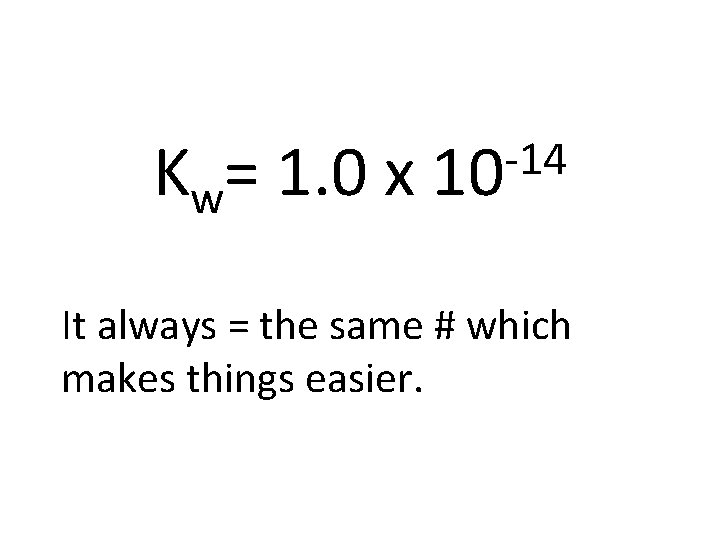
Kw= 1. 0 x -14 10 It always = the same # which makes things easier.
![1 0 x 14 10 H OH So if you know 1. 0 x -14 10 = + [H ][OH ] So if you know](https://slidetodoc.com/presentation_image_h2/bcdba7c8c698672cc514e0d9f926072c/image-6.jpg)
1. 0 x -14 10 = + [H ][OH ] So if you know [H+] or [OH-] you can plug it in and solve for the one you are missing.
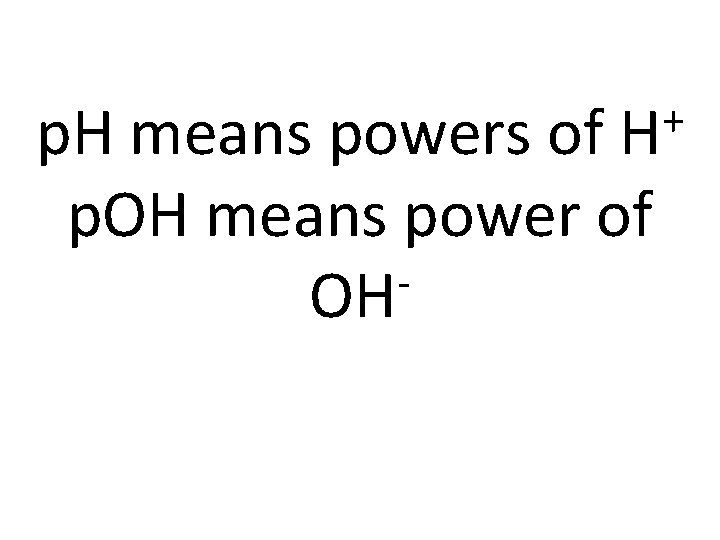
+ H p. H means powers of p. OH means power of OH

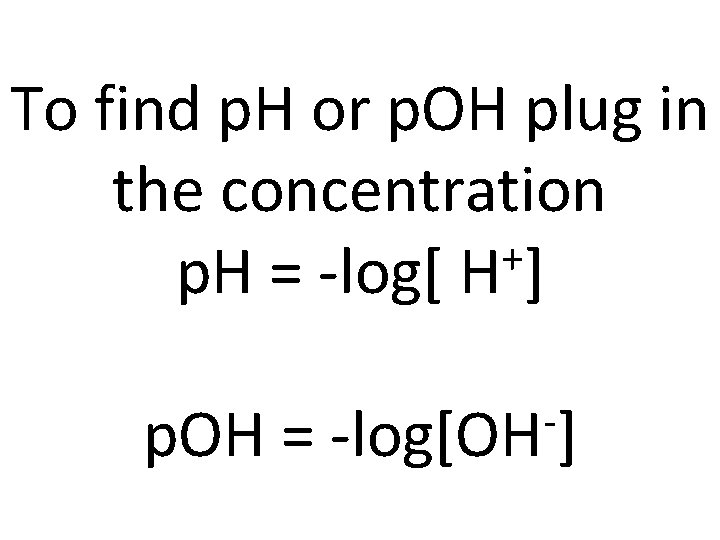
To find p. H or p. OH plug in the concentration + p. H = -log[ H ] p. OH = -log[OH ]

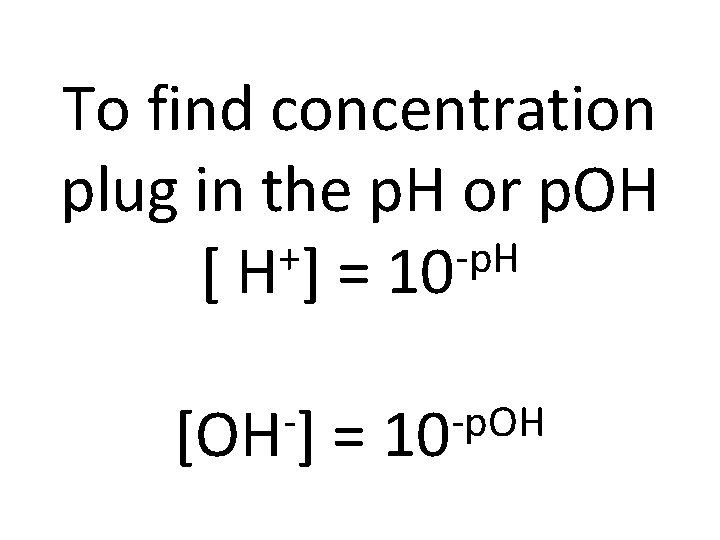
To find concentration plug in the p. H or p. OH + -p. H [ H ] = 10 [OH ] = -p. OH 10
![14 10 H OH 1 0 x log of everything -14 10 = + [H ][OH ] 1. 0 x -log of everything =](https://slidetodoc.com/presentation_image_h2/bcdba7c8c698672cc514e0d9f926072c/image-10.jpg)
-14 10 = + [H ][OH ] 1. 0 x -log of everything = 14 = p. H + p. OH

Naming Acids (a Refresher) • If anion is monoatomic, use hydro- + -ic – Ex: HCl = hydrochloric acid • If anion is polyatomic, look at name of ion and apply suffix: – Nitrate nitric acid – Sulfite sulfurous acid

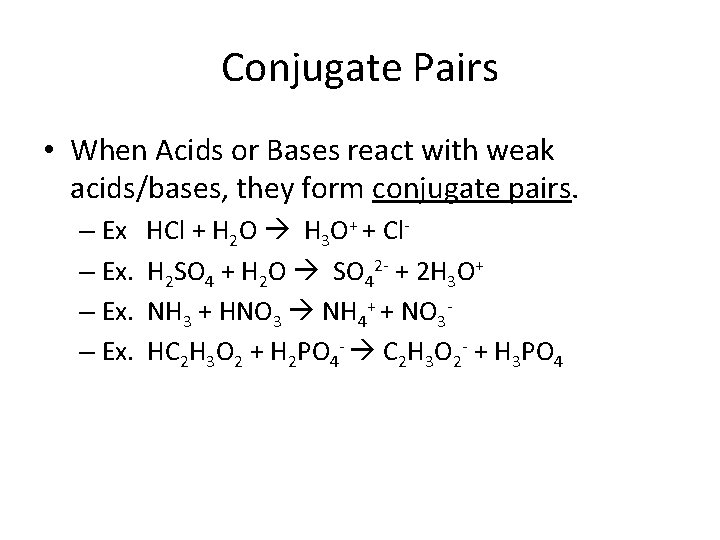
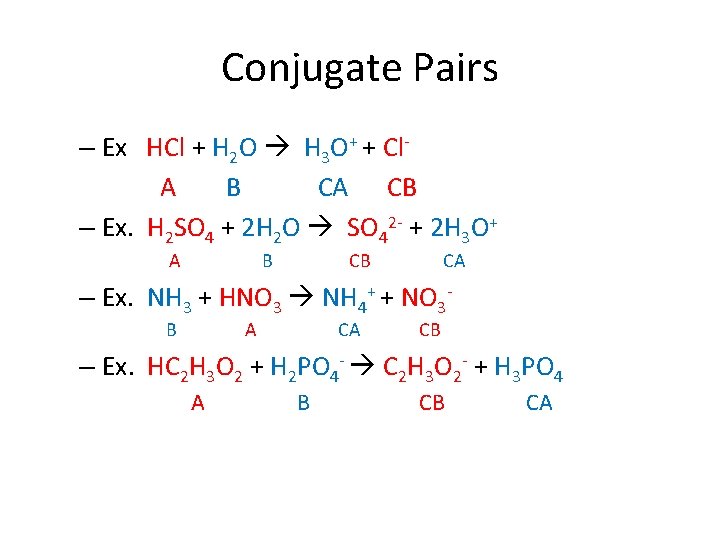
Conjugate Pairs • When Acids or Bases react with weak acids/bases, they form conjugate pairs. – Ex. HCl + H 2 O H 3 O+ + Cl. H 2 SO 4 + H 2 O SO 42 - + 2 H 3 O+ NH 3 + HNO 3 NH 4+ + NO 3 HC 2 H 3 O 2 + H 2 PO 4 - C 2 H 3 O 2 - + H 3 PO 4

Conjugate Pairs – Ex HCl + H 2 O H 3 O+ + Cl. A B CA CB – Ex. H 2 SO 4 + 2 H 2 O SO 42 - + 2 H 3 O+ A B CB CA – Ex. NH 3 + HNO 3 NH 4+ + NO 3 B A CA CB – Ex. HC 2 H 3 O 2 + H 2 PO 4 - C 2 H 3 O 2 - + H 3 PO 4 A B CB CA



Acid

Acid A substance that produces hydrogen ions in solution
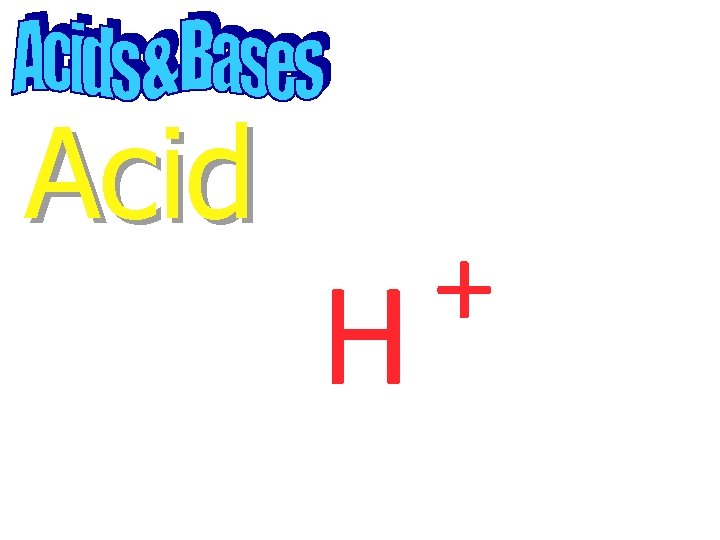
Acid + H

Acid A p. H less than 7

Acid A p. H less than 7 p. H stands for Powers of Hydrogen

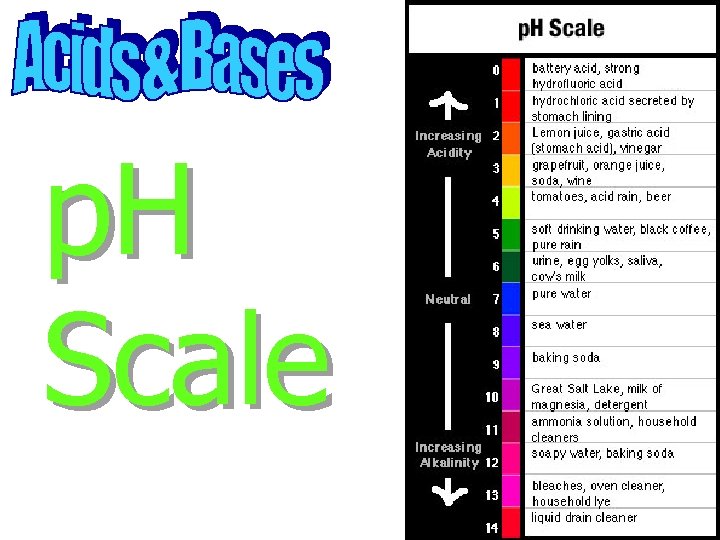
The p. H scale goes from 0 - 14

The p. H scale goes from 0 - 14 7 = Neutral

Acid 6 = Weakest Acid

Acid 6. 99 = Weakest Acid 0 = Strongest Acid

Acid

Acid - PROPERTIES

Acid - PROPERTIES Tastes sour

Acid - PROPERTIES Tastes sour Reacts with metal Turns litmus RED
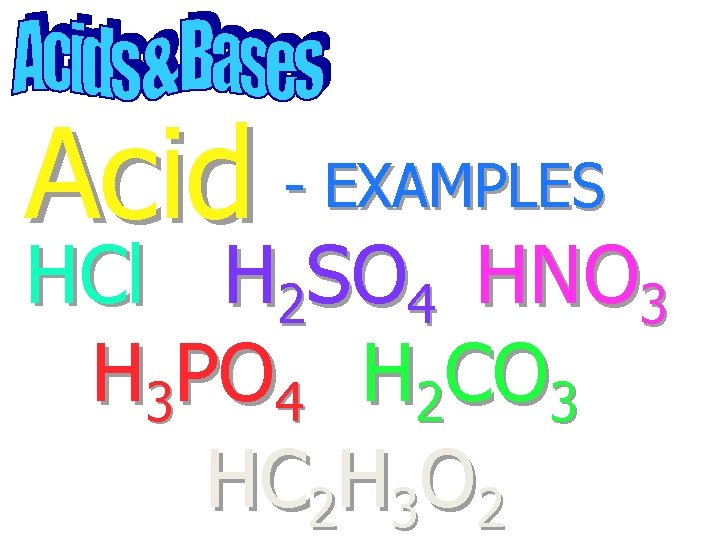
Acid - EXAMPLES HCl H 2 SO 4 HNO 3 H 3 PO 4 H 2 CO 3 HC 2 H 3 O 2


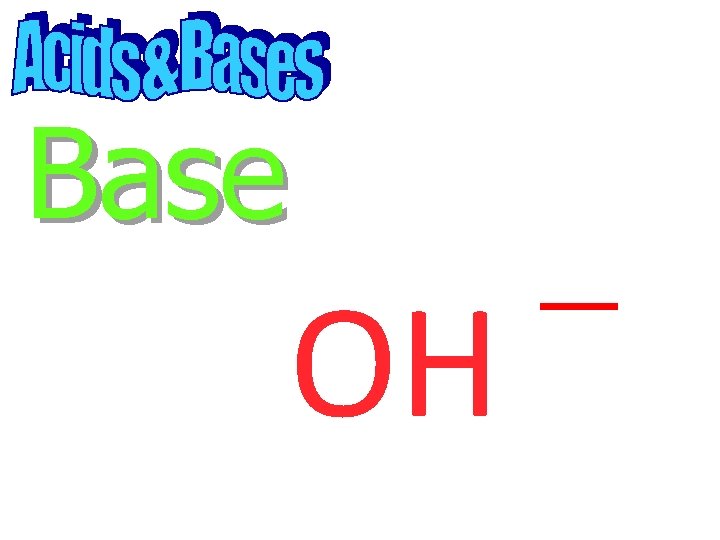
Base

Base A substance that produces hydroxide ions in solution

Base OH
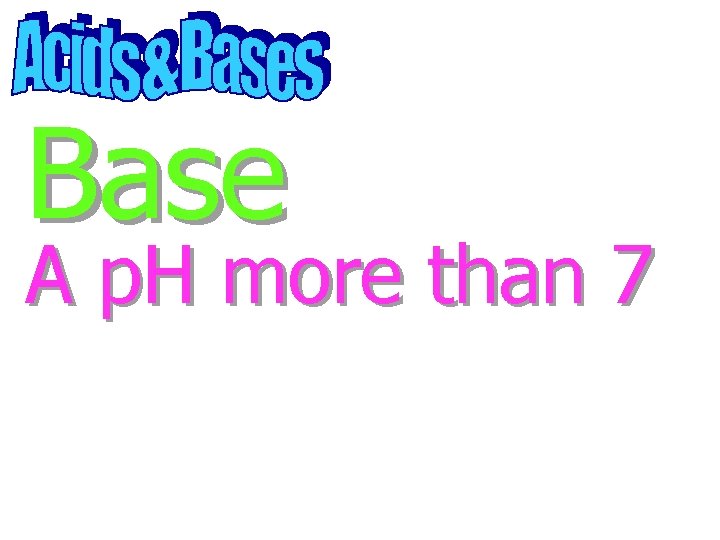
Base A p. H more than 7

Base

Base 8 = Weakest Base

Base 7. 01 = Weakest Base 14 = Strongest Base

Base

Base - PROPERTIES

Base - PROPERTIES Tastes bitter

Base - PROPERTIES Tastes bitter Feels slippery

Base - PROPERTIES Tastes bitter Feels slippery Turns litmus BLUE

Base

Base - EXAMPLES

Base Na. OH - EXAMPLES

Base - EXAMPLES Na. OH KOH

Base - EXAMPLES Na. OH KOH NH 3
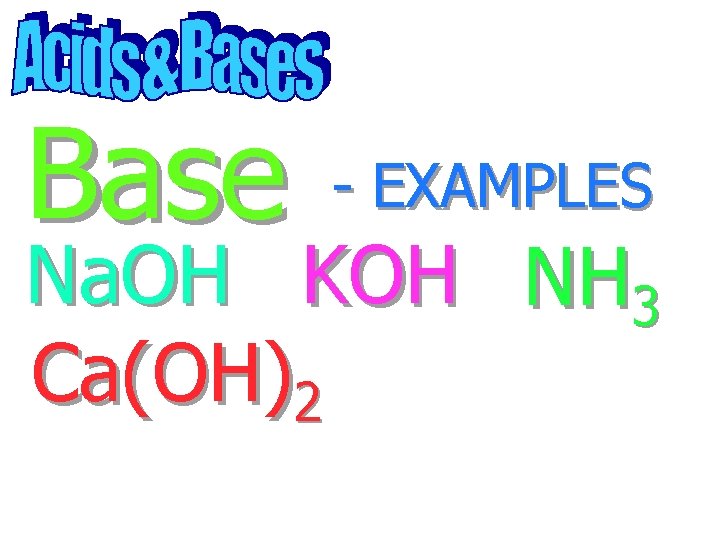
Base - EXAMPLES Na. OH KOH NH 3 Ca(OH)2
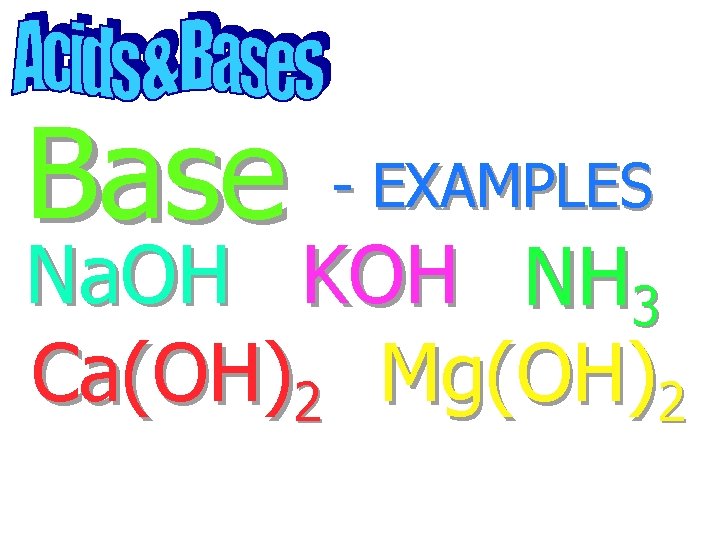
Base - EXAMPLES Na. OH KOH NH 3 Ca(OH)2 Mg(OH)2
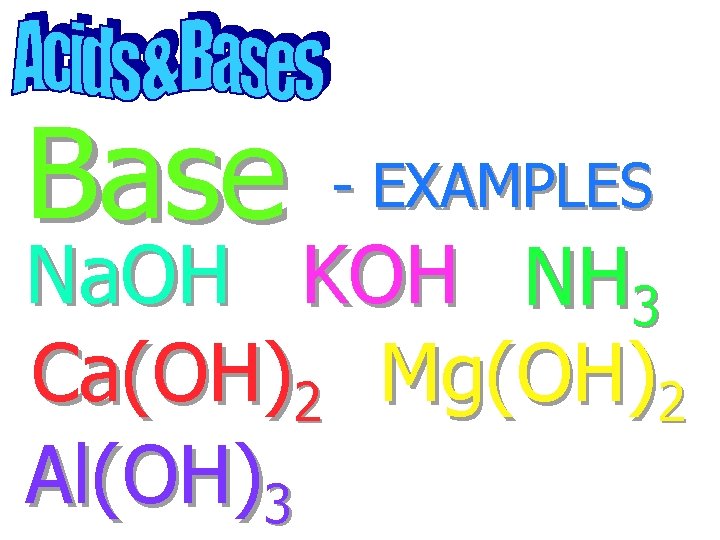
Base - EXAMPLES Na. OH KOH NH 3 Ca(OH)2 Mg(OH)2 Al(OH)3
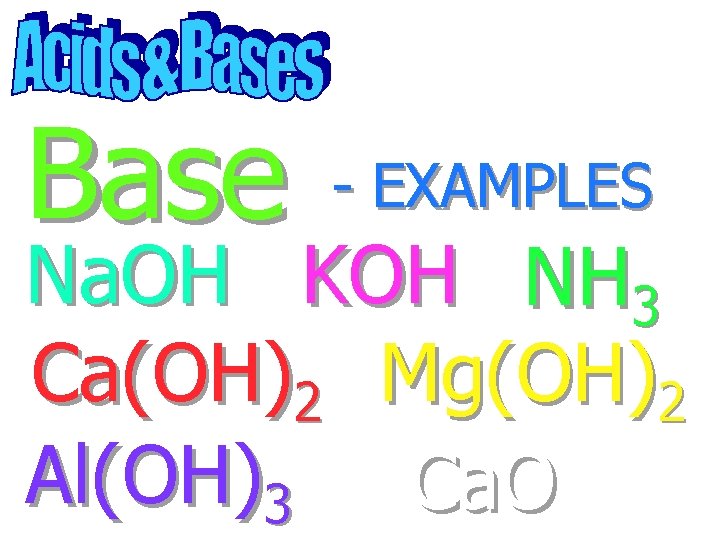
Base - EXAMPLES Na. OH KOH NH 3 Ca(OH)2 Mg(OH)2 Al(OH)3 Ca. O


p. H Scale

Reactions

Reactions Acid - Base
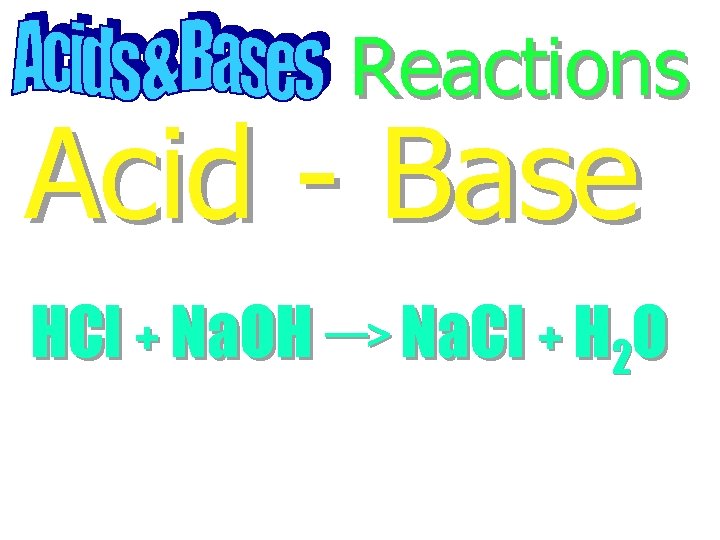
Reactions Acid - Base HCl + Na. OH Na. Cl + H 2 O

Reactions Acid - Base HCl + Na. OH Na. Cl + H 2 O Neutralization Reaction
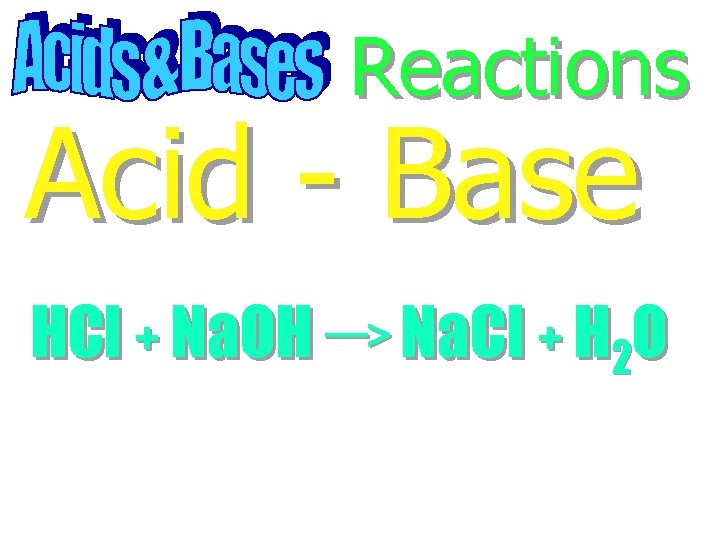
Reactions Acid - Base HCl + Na. OH Na. Cl + H 2 O
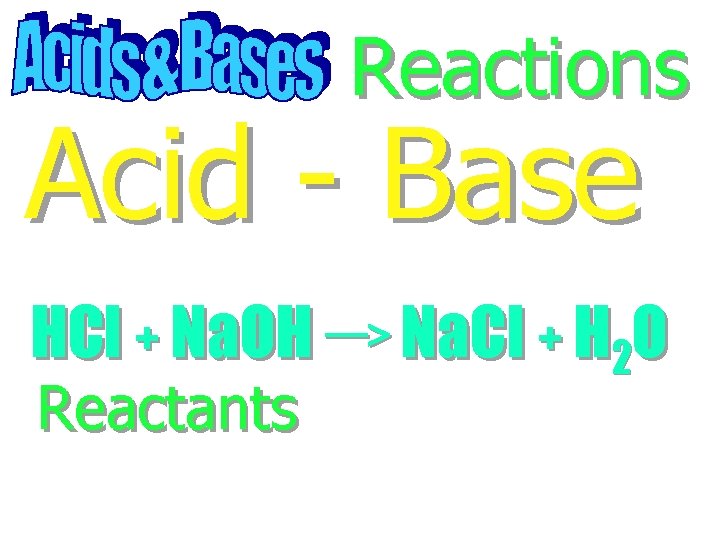
Reactions Acid - Base HCl + Na. OH Na. Cl + H 2 O Reactants

Reactions Acid - Base HCl + Na. OH Na. Cl + H 2 O Reactants Products

Reactions Acid - Base HCl + Na. OH Na. Cl + H 2 O Reactants Products Water + a salt
