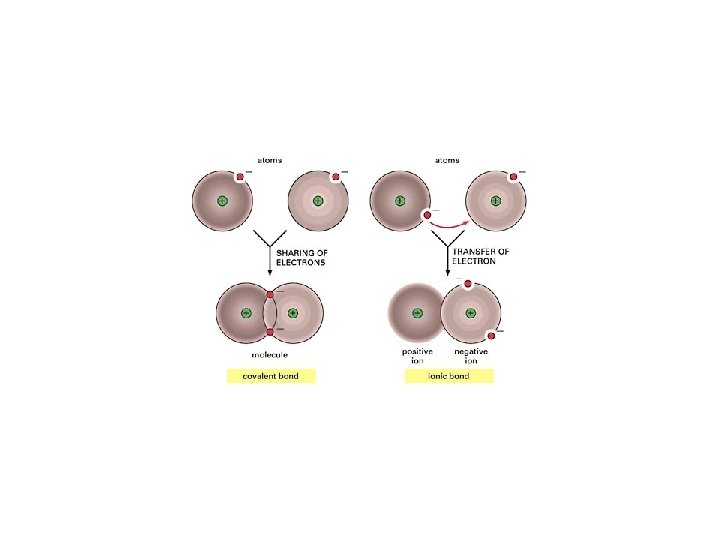
IONIC VS COVALENT COMPOUNDS PROPERTIES OF MOLECULES Exist









- Slides: 32

IONIC VS. COVALENT COMPOUNDS

PROPERTIES OF MOLECULES • Exist as solids, liquids or gases at room temperature • Solids are often soft and waxy • Liquids and solids often are volatile and can evaporate readily [ie. Perfume or mothballs] • Low melting and boiling points • Low solubility in water and other polar (charged) solvents • Soluble in non-polar solvents such as chloroform or ether • As solids, liquids or solutions are non-conductors of electricity


PROPERTIES OF FORMULA UNITS • Crystalline • Solids at room temperature • High melting points and boiling points • Hard but brittle • Soluble in water • Electrolytes when dissolved in water [conduct electricity] • As solids are non conductors of electricity • As liquids conduct electricity




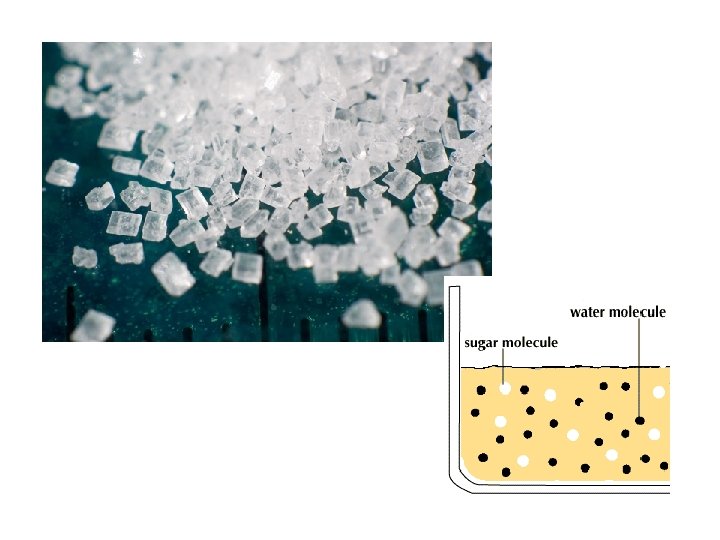
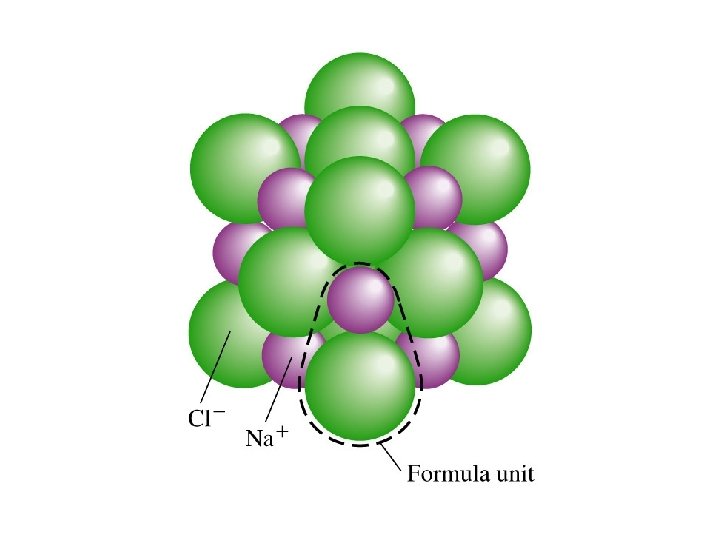
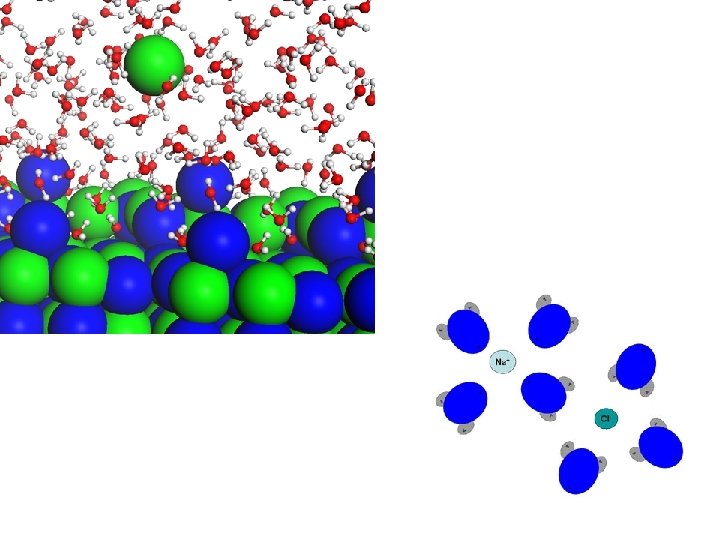
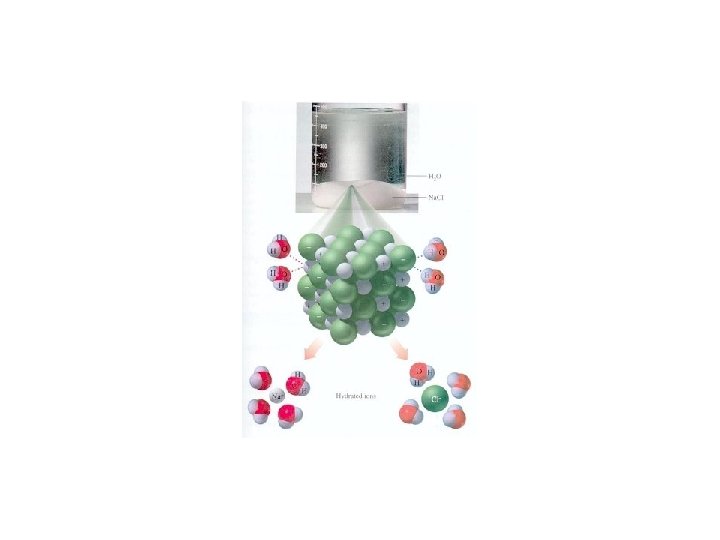
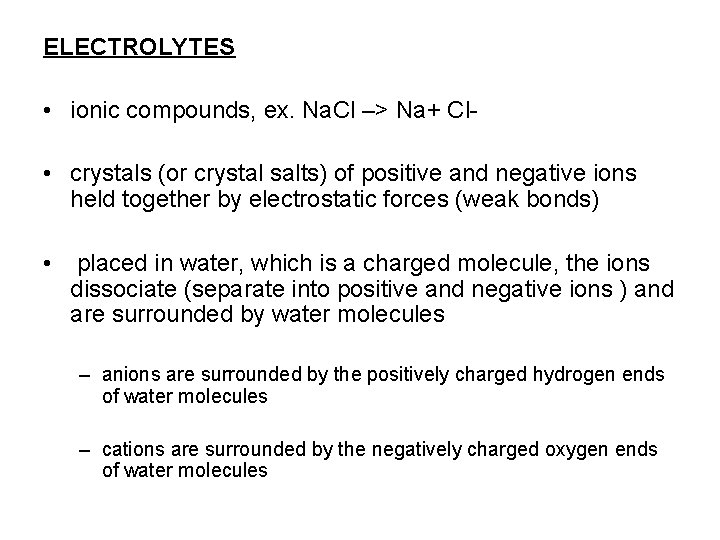
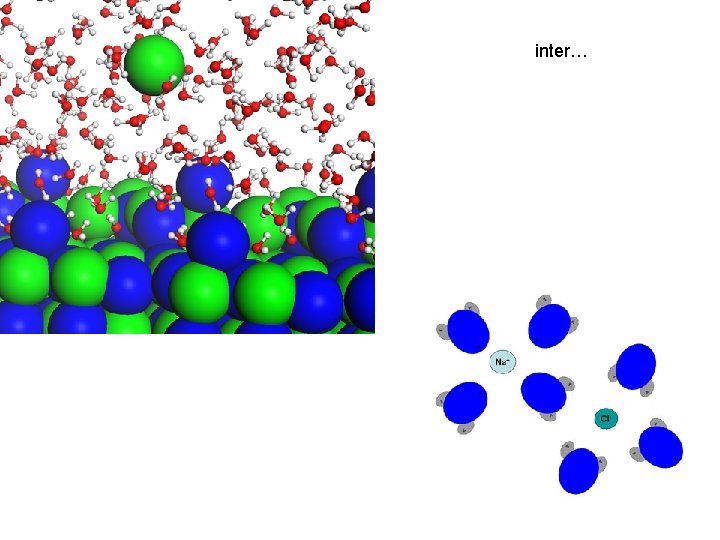
ELECTROLYTES • ionic compounds, ex. Na. Cl –> Na+ Cl • crystals (or crystal salts) of positive and negative ions held together by electrostatic forces (weak bonds) • placed in water, which is a charged molecule, the ions dissociate (separate into positive and negative ions ) and are surrounded by water molecules – anions are surrounded by the positively charged hydrogen ends of water molecules – cations are surrounded by the negatively charged oxygen ends of water molecules

conductivity • the ability of a substance to conduct electricity; a physical property of matter • some substances can and others can't electrolyte: a compound that, when dissolved in water, produces a solution that conducts electricity: ionic substances nonelectrolyte: a compound that, when dissolved in water, does not produce a solution that conducts electricity: covalent substances

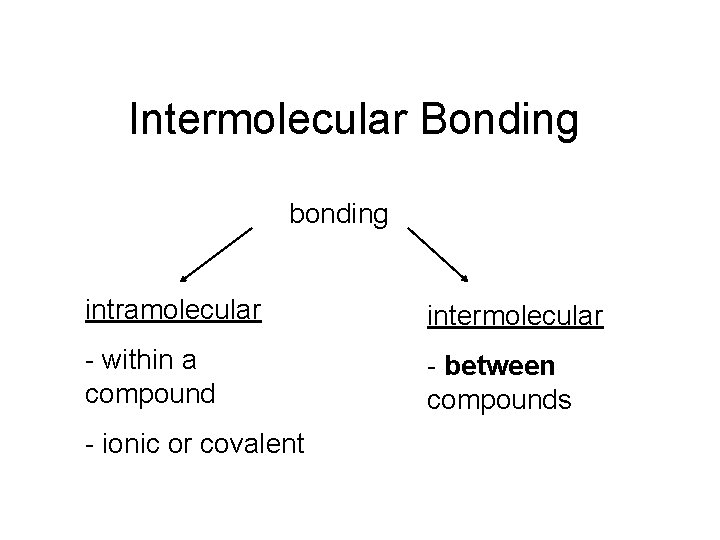
Intermolecular Bonding bonding intramolecular intermolecular - within a compound - between compounds - ionic or covalent

intra…


inter…

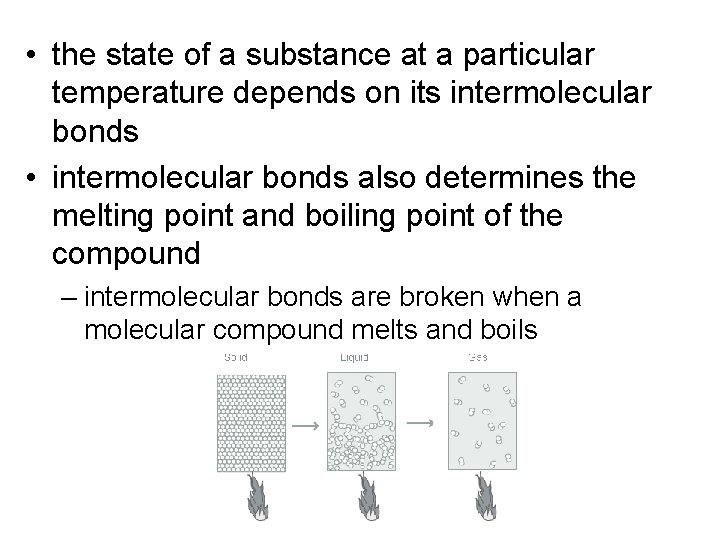
• the state of a substance at a particular temperature depends on its intermolecular bonds • intermolecular bonds also determines the melting point and boiling point of the compound – intermolecular bonds are broken when a molecular compound melts and boils


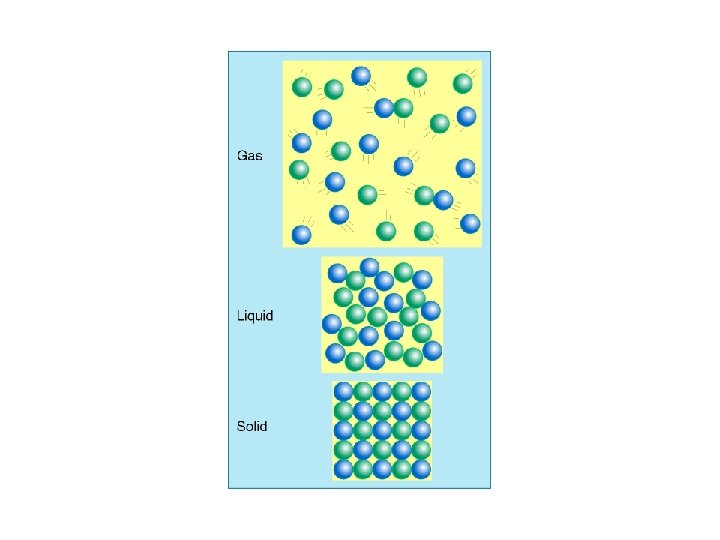
• at room temperature, a substance is: – a solid if its compounds are held closely together (many intermolecular forces) – a liquid if they are held further apart (some intermolecular forces) – a gas if there is little attraction between them (few intermolecular forces)

• molecules that have a difference in charge distribution will hold together strongly, – ex. – recall: electronegativity - the attraction an atom feels for electrons - increases up and across the per table


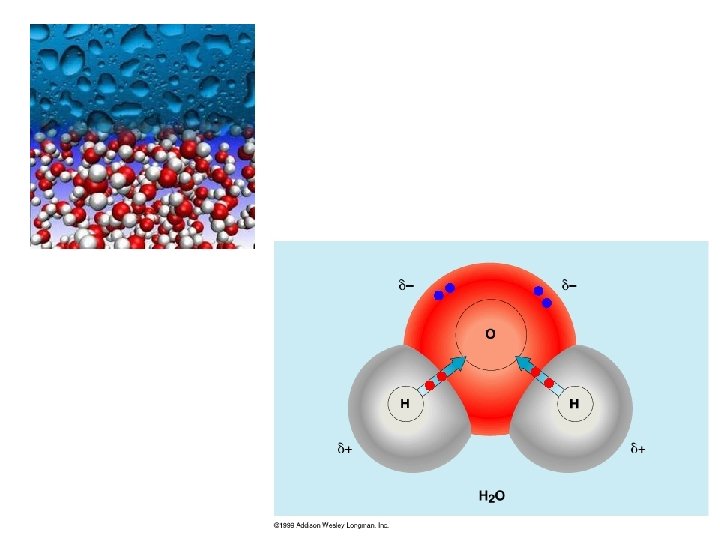
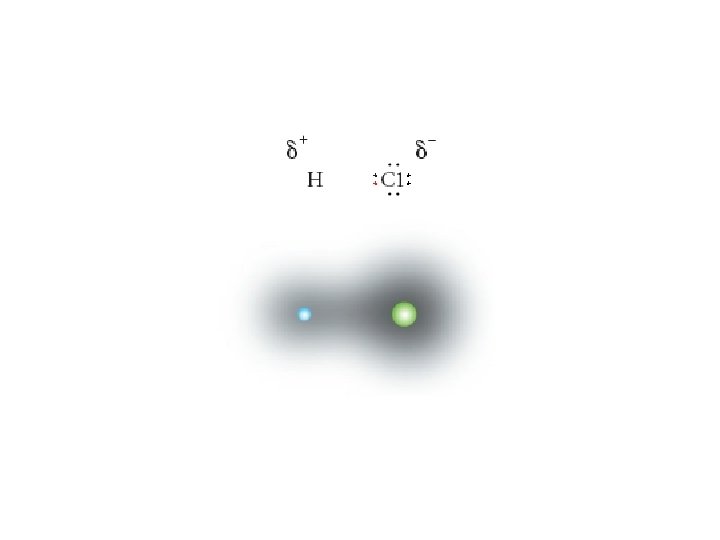
polar molecules • molecules that have a positively charged end a negatively charged end • this will depend on the electronegativity of the atoms involved polar covalent bonding • when an electron pair is not shared equally, there is a localized negative charge around one atom, represented by the symbol δ-, while the other atom is more positively charged, δ+ • a polar covalent bond has a slightly negative end a slightly positive end, ex. H 2 O, HCl (g)


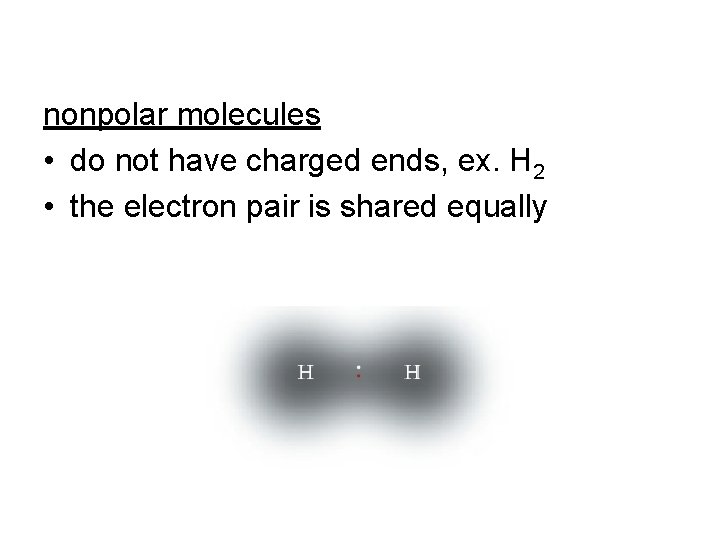
nonpolar molecules • do not have charged ends, ex. H 2 • the electron pair is shared equally

Polarity of a molecule depends on: 1. the presence of polar covalent bonds 2. the three-dimensional shape (geometry) of the molecule

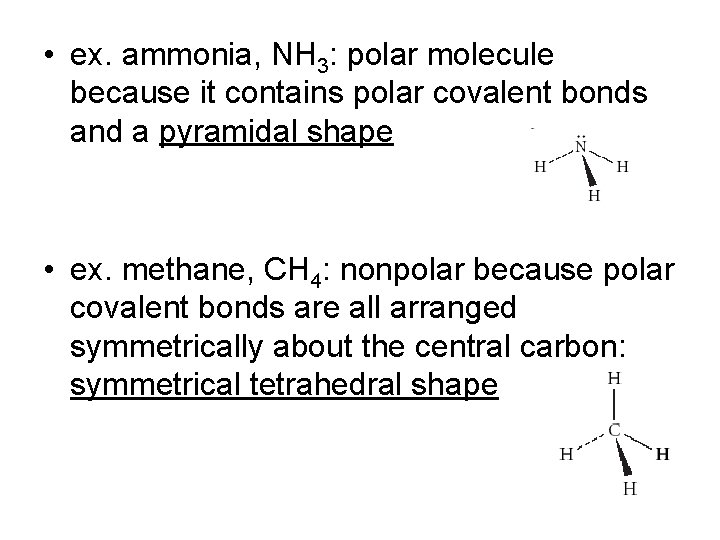
• ex. ammonia, NH 3: polar molecule because it contains polar covalent bonds and a pyramidal shape • ex. methane, CH 4: nonpolar because polar covalent bonds are all arranged symmetrically about the central carbon: symmetrical tetrahedral shape

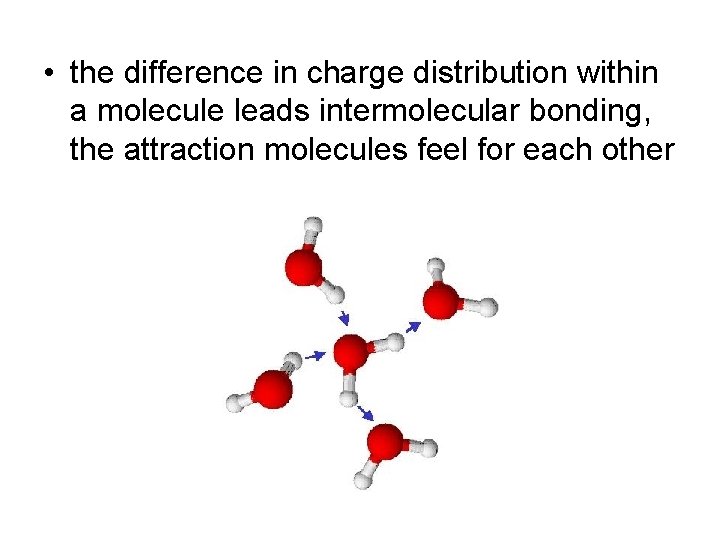
• the difference in charge distribution within a molecule leads intermolecular bonding, the attraction molecules feel for each other

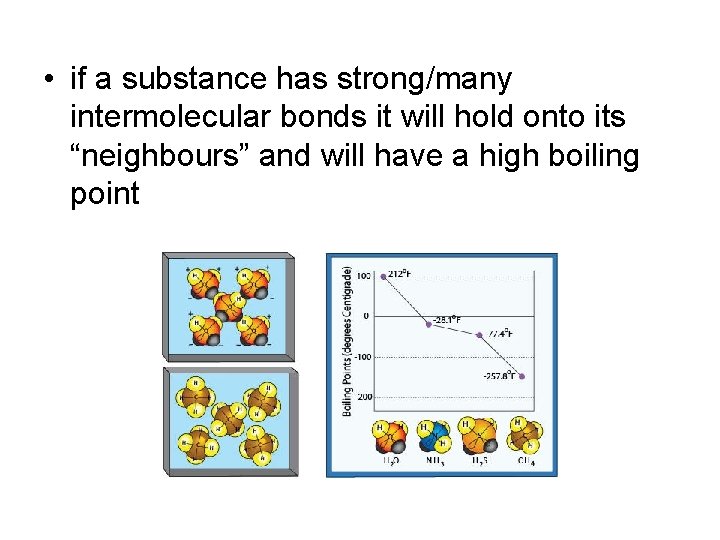
• if a substance has strong/many intermolecular bonds it will hold onto its “neighbours” and will have a high boiling point

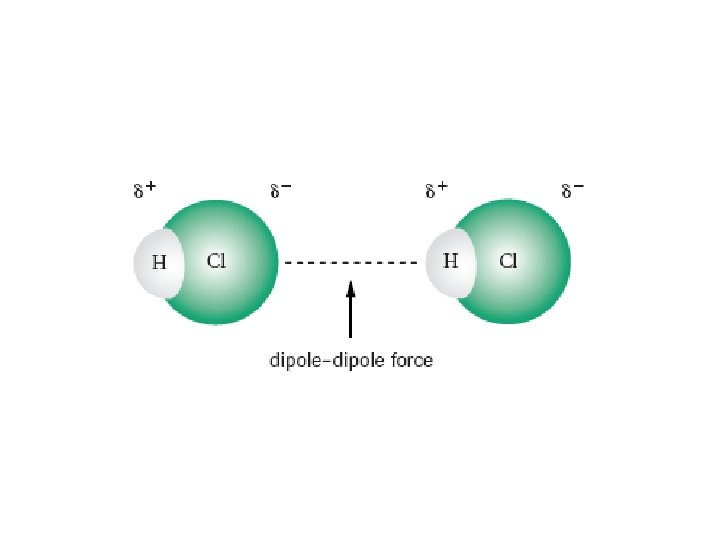
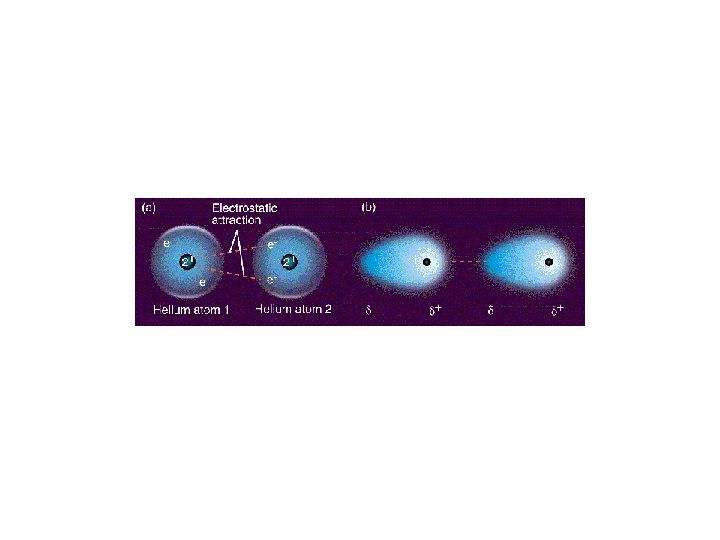
Types of Intermolecular Bonds van der Waals forces • weak forces of attraction between molecules, DDF and LDF 1. dipole–dipole force (DDF) • occurs between polar molecules, such as hydrogen chloride, HCl • the slightly positive end of one hydrogen chloride molecule is attracted to the slightly negative end of a neighbouring hydrogen chloride molecule


2. London dispersion force (LDF) • weakenst type, an intermolecular force of attraction that forms between atoms of neighbouring molecules as a result of a temporary imbalance in the position of the atoms’ electrons • forms between all molecules, polar and nonpolar – the side of the atoms with more electrons develops a temporary negative charge, and the side with fewer electrons develops a temporary positive charge; if same happens to neighbouring molecule they attract each other – since electrons move quickly, the dipole lasts for only a fraction of a second


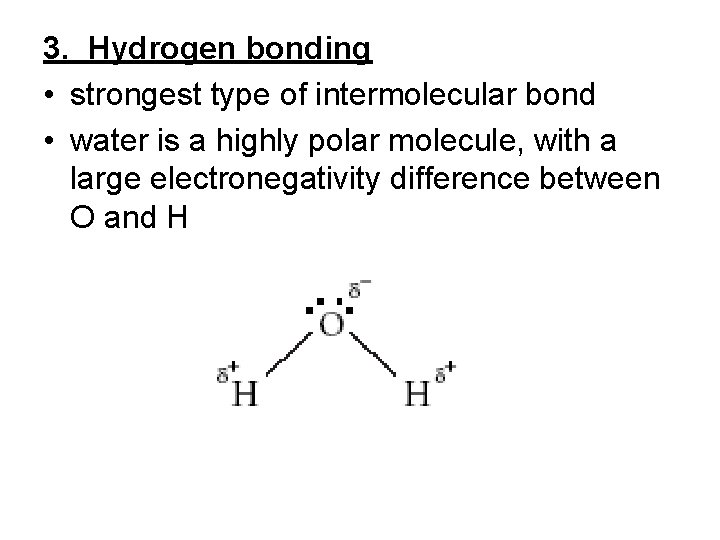
3. Hydrogen bonding • strongest type of intermolecular bond • water is a highly polar molecule, with a large electronegativity difference between O and H

• since oxygen has the higher electronegativity, the electrons spend more time around oxygen than they do around hydrogen, therefore the oxygen end of a water molecule has a slightly negative charge while the hydrogen end of the molecule is slightly positive • the arrangement of atoms and bonds forms a bent shape

Homework 1. List the characteristics of covalent and ionic compounds. 2. How are solids/liquids/gases different from each other. 3. What is polarity? 4. What makes a molecule polar? 5. What are the different types of intermolecular bonds, from strongest to weakest?