Ionic Molecular Compounds Ionic Bonding Susan Baird Dori

Ionic & Molecular Compounds Ionic Bonding Susan Baird Dori Delaney Cindy Rothwell Ionic & Molecular Compounds

Ionic Bonding When an atom gains or loses electrons, the atom is no longer neutral. It has become an ion. An ion is a particle or group of particles with a positive (+) or negative (-) charges. Return Home Site Ionic & Molecular Compounds

Ionic Bonding Cations are positively charged ions. (Na +) Metals lose all valence electrons forming cations. Anions are negatively charged ions. (Cl-) Nonmetals gain electrons to fill their outer valence shells By gaining electrons, nonmetals form anions. Return Home Site Ionic & Molecular Compounds

Ionic Bonding Examples of common cations and anions Common Cations hydrogen H+ sodium Na+ potassium K+ calcium Ca+2 magnesium Mg+2 Common Anions hydroxide OHchloride Clsulfide S-2 Oxygen O-2 Nitrogen N -3 Bromine Br - Return Home Site Ionic & Molecular Compounds

Ionic Bonding Knowledge of the charges of ions is crucial to knowing the formulas of the compounds formed. Alkali Metals (1 st column elements) form "+1" ions such as Na+ and Li+ Alkaline earth metals (2 nd column elements) form "2+" ions such as Mg 2+ and Ba 2+ Halogens (7 th column elements) form "-1" ions such as Cl 1 - and I 1 Return Home Site Ionic & Molecular Compounds
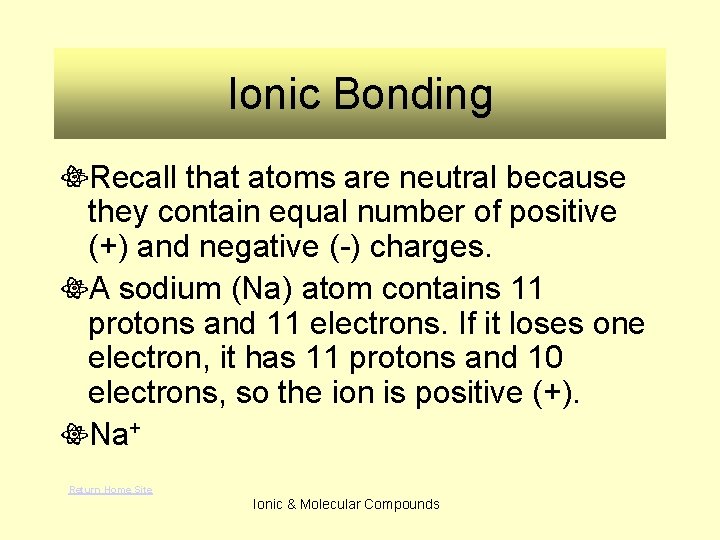
Ionic Bonding Recall that atoms are neutral because they contain equal number of positive (+) and negative (-) charges. A sodium (Na) atom contains 11 protons and 11 electrons. If it loses one electron, it has 11 protons and 10 electrons, so the ion is positive (+). Na+ Return Home Site Ionic & Molecular Compounds
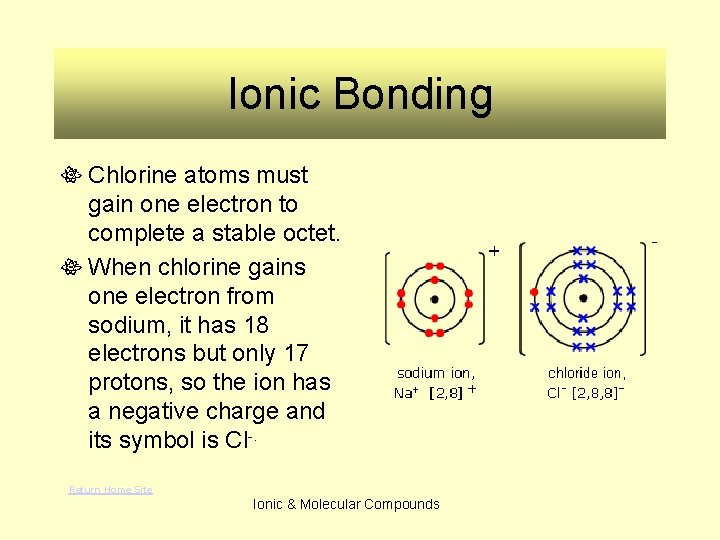
Ionic Bonding Chlorine atoms must gain one electron to complete a stable octet. When chlorine gains one electron from sodium, it has 18 electrons but only 17 protons, so the ion has a negative charge and its symbol is Cl-. Return Home Site Ionic & Molecular Compounds

Ionic Bonding Ionic Compounds are formed when positive (+) and negative (-) ions attract each other after the electron rearrangement happens. This attraction is called an IONIC BOND. The attraction is not only strong but also the same in all directions. It extends from one ion to the next throughout an ionic compound. Return Home Site Ionic & Molecular Compounds
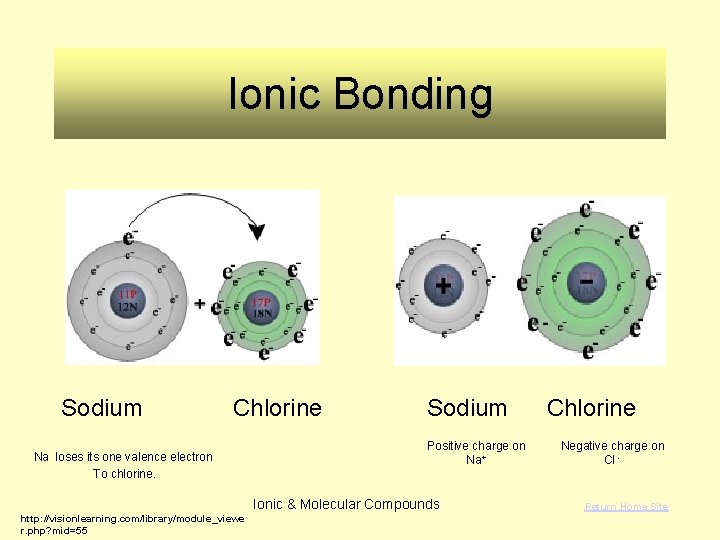
Ionic Bonding Sodium Chlorine Na loses its one valence electron To chlorine. Sodium Positive charge on Na+ Ionic & Molecular Compounds http: //visionlearning. com/library/module_viewe r. php? mid=55 Chlorine Negative charge on Cl - Return Home Site
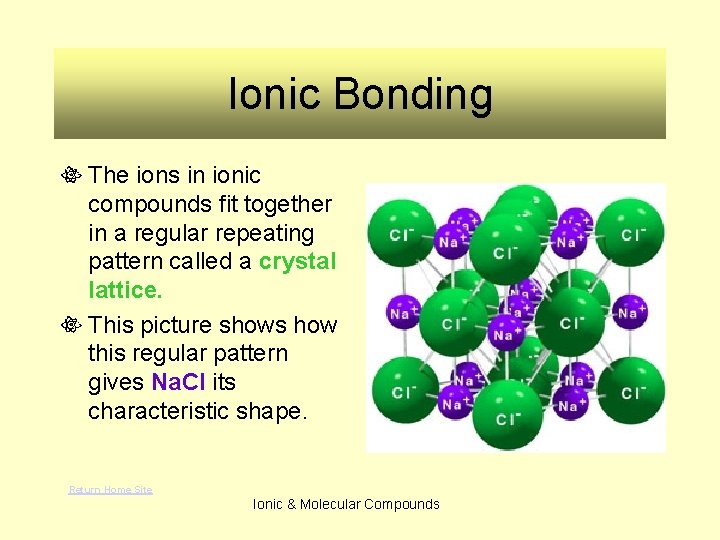
Ionic Bonding The ions in ionic compounds fit together in a regular repeating pattern called a crystal lattice. This picture shows how this regular pattern gives Na. Cl its characteristic shape. Return Home Site Ionic & Molecular Compounds

Electronegativity and Ionic Compounds Electronegativity: the relative attraction for the bonding electrons by the atoms in a bond. Electronegativity expresses the pulling ability of two atoms. It was first suggested by American chemist Linus Pauling (1901– 1994). Pauling proposed the name "electronegativity" for this property of atoms. Return Home Site Ionic & Molecular Compounds

Electronegativity continued Two atoms with the same or similar electronegativities will end up sharing electrons between them in a covalent bond. Two atoms with very different electronegativities will form ionic bonds. (http: //www. scienceclarified. com/Ca-Ch/Chemical-Bond. html) Return Home Site Ionic & Molecular Compounds

Characteristics of Ionic Compounds High melting points (Na. Cl 801°C) Due to crystalline shapes. High boiling points (Na. Cl 1413°C) Dissolves in water to give aqueous solutions that conduct electricity. Ionic bonds form between metals and nonmetals. In naming simple ionic compounds, the metal is always first, the nonmetal second (e. g. , sodium chloride). Ionic & Molecular Compounds Return Home Site

Ionic Bonding Determining the charge on an ion, for the main group of elements, use the periodic table. These elements will gain or lose electrons to fill or empty their valence shells. (Octet Rule) Return Home Site Ionic & Molecular Compounds

Ionic Bonding For metals, the charge will equal to their Main Group Number e. g. Na is in Group 1 A and forms a 1+ ion, Mg is in group 2 A and forms a 2+ ion, Al is in group 3 A and forms 3+ ion. (If you need a refresher, review the valence electron power point and the metals, nonmetals and metalloids power point. ) Return Home Site Ionic & Molecular Compounds

Ionic Bonding For nonmetals, the charge they will form is equal to their Main Group Number minus 8. For example, F forms a – 1 ion, Group 7 A – 8 = -1, oxygen forms – 2, Group 6 A – 8 = -2. Remember, that the charge that an element forms is the same for its entire group. Ionic & Molecular Compounds Return Home Site
Ionic Bonding Videos A video describing Ionic & Covalent Bonds. Difference between Ionic, Polar, and Nonpolar bonds. Sodium and Chlorine ionic bond. Ionic & Molecular Compounds Return Home Site

Ionic Bonding Test your knowledge. Visit this interactive website, test your abilities, and have fun! Bonds and the Octet Rule. Practice valence electrons. Worksheets and practice Ionic & Molecular Compounds Return Home Site

Ionic Compounds Try and form compounds by combining the ions. When you make the compounds the total number of positive charges has to equal the total number of negative charges in a compound. Try forming compounds, from these elements, Na, Mg, O, Cl, Mg, F, Ionic & Molecular Compounds Return Home Site

Ionic Compounds Answers: Na. Cl Mg. O Na 2 O Mg. F 2 Na. F Mg. F 2 Ionic & Molecular Compounds Return Home Site

Ionic Bonding Please click on the following underlined links to return to the Ionic & Molecular Home page or Ionic Bonding page. Home Page Ionic Bonding Ionic & Molecular Compounds Return Home Site
- Slides: 21